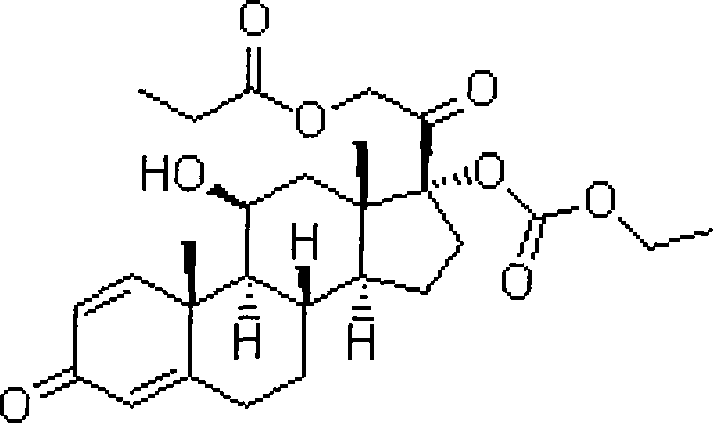
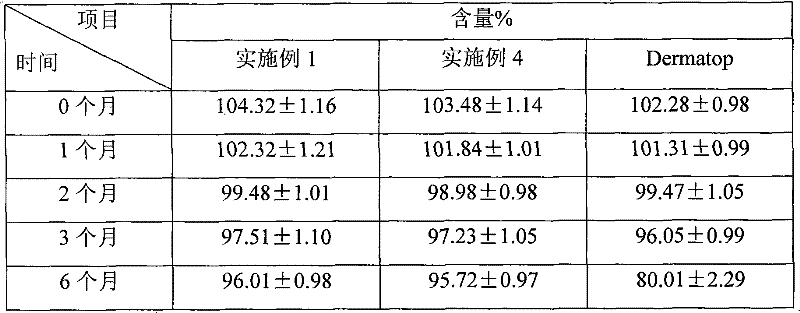
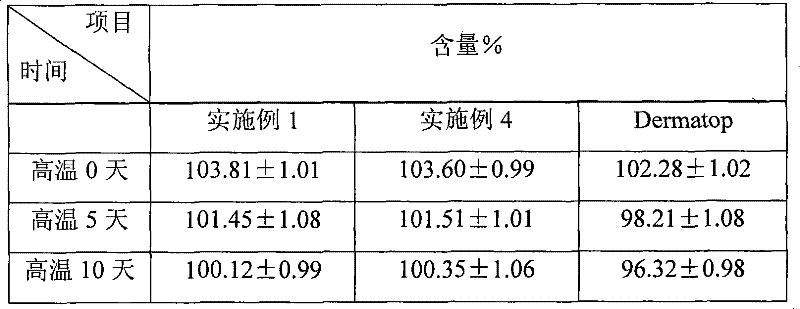
prednicate liposomal cream
A technology of prednica ester and prednica ester, which is applied in the field of creams containing prednica ester liposomes, can solve the problem that glucocorticoids are difficult to be loaded into liposomes and cannot fully exert skin external liposomes. Advantages of drug delivery, influence on drug penetration and absorption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Recipe for the oil phase:
[0042] White Vaseline 100g Stearyl Alcohol 30g Liquid Paraffin 50g Glyceryl Monostearate 20g Pingpinga-2020g Methylparaben 0.5g Propylparaben 0.05g
[0043] The formula of liposome solution:
[0044] Prednicate 1g Soy lecithin 20g Cholesterol 2g Disodium ethylenediaminetetraacetic acid (EDTA-2Na) 1g α-tocopherol 1g
[0045] Buffer the liposome solution to pH=6.5 in phosphate buffer
[0046] Glycerin 50g
[0047] Distilled water to 1000g (based on the total weight of the composition)
[0048] Preparation:
[0049] (1) Preparation of oil phase
[0050] Take the prescribed amount of oil phase ingredients and heat to 70°C, add paraben dissolved in an appropriate amount of organic solvent, then take the prescribed amount of moisturizer and heat it to the same temperature and slowly add it to the oil phase, and stir until it is uniform while adding. have to.
[0051] (2) Preparation of liposome solution
[0052] Prednicate, phospholipids, c...
Embodiment 2
[0056] Oil Phase Formula:
[0057] White Vaseline 120g Stearyl Alcohol 50g Liquid Paraffin 50g Glyceryl Monostearate 40g Pingpingjia a-20 20g Methylparaben 0.5g Propylparaben 0.05g
[0058] The liposome solution formula is:
[0059] Prednicate 0.5g Hydrogenated soybean lecithin 10g Cholesterol 1g Disodium edetate 1g Glycerin 40g
[0060] Buffer the liposome solution to pH=6.5 in phosphate buffer
[0061] Distilled water to 1000g (based on the total weight of the composition)
[0062] Prepare 0.05% prednicardate liposome emulsifiable cream as the compound method of embodiment 1.
Embodiment 3
[0064] Oil Phase Formula:
[0065] White Vaseline 60g Cetyl Alcohol 40g Octadecanol 40g Liquid Paraffin 90g Glyceryl Monostearate 20g Pingpinga a-20 40g Methylparaben 0.5g Propylparaben 0.05g
[0066] Liposome Solution Formula:
[0067] Prednicate 0.25g Egg yolk lecithin 3g Cholesterol 0.6g
[0068] Buffer the liposome solution to pH=6.5 in phosphate buffer
[0069] Distilled water to 1000g (based on the total weight of the composition)
[0070] Prepare 0.025% prednicardate liposome emulsifiable cream as the compound method of embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com