Method for preparing photographic-grade silver nitrate through one-time crystallization
A silver nitrate, disposable technology, applied in chemical instruments and methods, silver compounds, silver compounds and other directions, can solve the problems that the reaction speed is difficult to control, the photographic grade silver nitrate cannot be reached, the reaction conditions are high, and the production cost is low. , The effect of good sensitivity and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
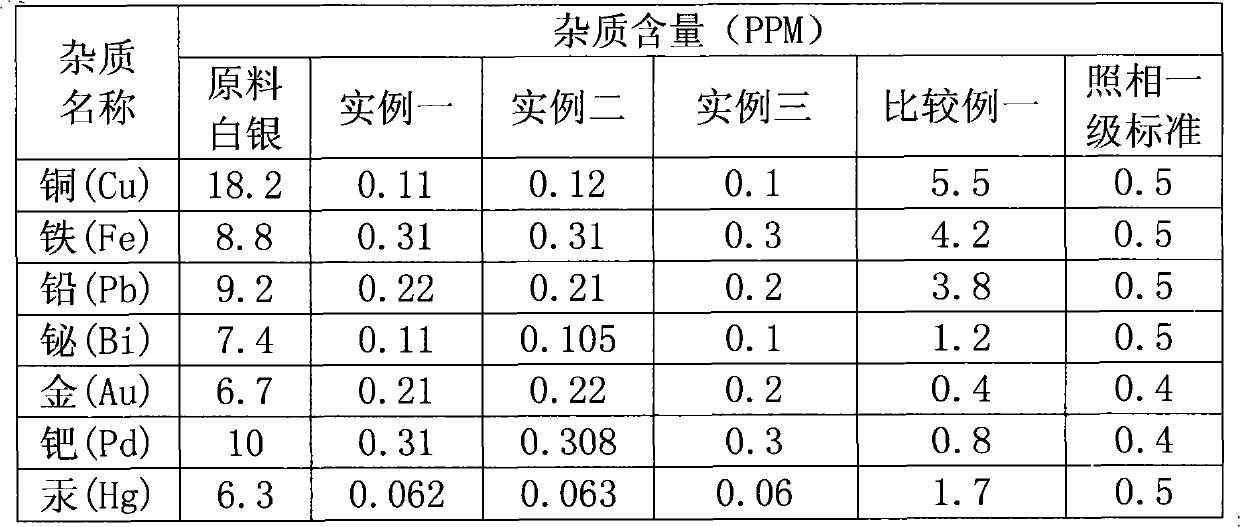
Embodiment 1
[0019] A kind of disposable crystallization prepares the method for photographic grade silver nitrate, it comprises the following steps:
[0020] a. Take the national standard 1 # 13 kilograms of silver ingots are put into the reactor, and then 11 liters of 61% concentrated nitric acid are added to the reactor;
[0021] b. Heating the reactor to increase the temperature of the solution gradually. When it reaches 60°C, the reaction gradually becomes violent. Continue heating to bring the solution temperature to 81°C to allow it to fully react. After the reaction is over, continue heating for 2 hours until it is invisible Until the nitrogen oxide overflows, the pH value of the solution is in the range of 1 to 2;
[0022] c. transfer the above-mentioned prepared solution into an impurity removal container with a stirring device, stir at a speed of 50 rpm, add silver oxide in the process of stirring, and the addition is 5 grams. When the pH of the solution is 7.6, stop stirring,...
Embodiment 2
[0026] A kind of disposable crystallization prepares the method for photographic grade silver nitrate, it comprises the following steps:
[0027] a. Take the national standard 1 # 178 kilograms of silver ingots are put into the reactor, and then 16 liters of 69% concentrated nitric acid are added to the reactor;
[0028] b. Heat the reactor to increase the temperature of the solution gradually. When it reaches 60°C, the reaction will gradually become violent. Continue heating until the solution temperature reaches 90°C to make it fully react. After the reaction is over, continue heating for 3 hours until the Until the nitrogen oxide overflows, the pH value of the solution is in the range of 1 to 2;
[0029] c. transfer the solution prepared above into the impurity removal container with a stirring device, stir at a speed of 50 rpm, add silver oxide in the process of stirring, and the addition is 70 grams. When the pH of the solution is At 8.4, stop stirring, and filter after...
Embodiment 3
[0033] A kind of disposable crystallization prepares the method for photographic grade silver nitrate, it comprises the following steps:
[0034] a. Take the national standard 1 # 15 kilograms of silver ingots are put into the reactor, and then 13.5 liters of 65% concentrated nitric acid are added to the reactor;
[0035] b. Heat the reactor to increase the temperature of the solution gradually. When it reaches 60°C, the reaction will gradually become violent. Continue heating to bring the solution temperature to 80-90°C to make it fully react. After the reaction is over, continue heating for 2-3 hours , until no overflow of nitrogen oxides can be seen, so that the pH value of the solution is in the range of 1 to 2;
[0036] c. transfer the solution prepared above into the impurity removal container with a stirring device, stir at a speed of 50 rpm, add silver oxide in the process of stirring, and the addition is 60 grams. When the pH of the solution is At 8 o'clock, stop st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com