Cathearanthus alkaloid
A technology of periwinkle and alkaloids, which can be applied in drug combinations, compounds of group 5/15 elements of the periodic table, organic chemistry, etc., can solve the problems of high neurotoxic side effects and low anti-tumor activity, and achieve good anti-tumor activity , low neurotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
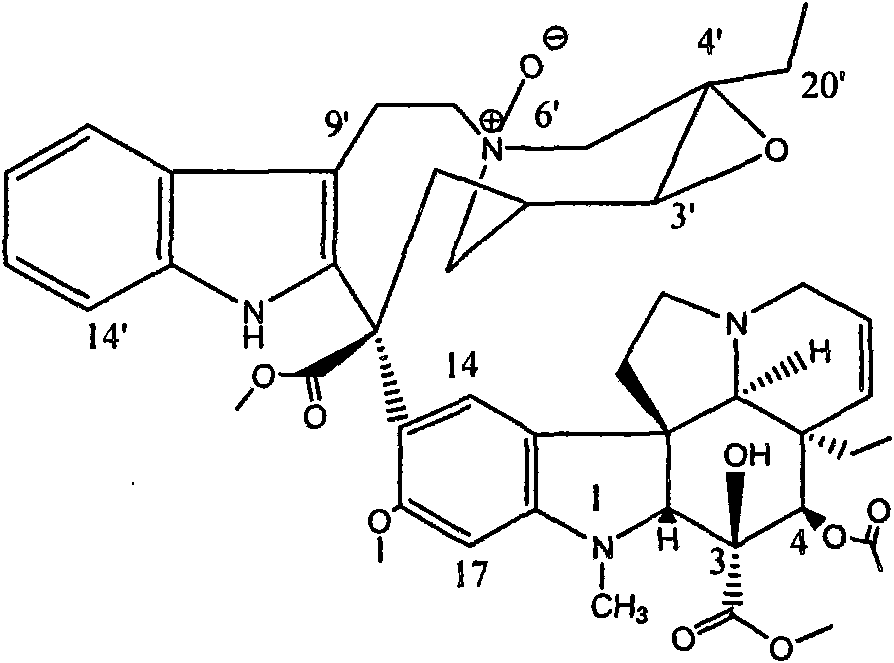
[0024] Obtaining of Compound A1
[0025] 6'-N oxidation-epoxy vinblastine (6'-N b -oxide-leurosine) at the 3-position for semi-synthetic modification, the original -COOCH at the 3-position 3 Replace with -COOCH 2 C 6 h 5 , whose structure is:
[0026]
[0027] Semi-lethal dose (IC 50 ) Experimental results:
[0028] IC 50 Value (μM / L)
[0029] cancer cell line
He La
7.3
16
A549
2.9
3.6
DLD-1
10.7
14
MX-1
1.6
1.8
OVCAR-3
1.5
2.0
[0030] Mouse animal experiment results (the number of mice with abnormal conditions):
[0031]
[0032] Activity experiments showed that the semi-lethal dose (IC 50 ) values are all less than vinblastine, indicating that it has better antitumor activity than vinblastine; meanwhile, the mouse animal experiment results show that the number of mice with abnormal conditions is significantly lowe...
Embodiment 2
[0034] Obtaining of compound B1
[0035] 6'-N oxidation-epoxy vinblastine (6'-N b -oxide-leurosine) at the 3-position for semi-synthetic modification, the original -COOCH at the 3-position 3 Replace with -COOCH 2 C 6 h 5 , while the original -OCOCH at the 4-position 3 Substituted by -OH, its structure is:
[0036]
[0037] Semi-lethal dose (IC 50 ) Experimental results:
[0038] IC 50 Value (μM / L)
[0039] cancer cell line
Compound B1
He La
8.3
16
A549
3.0
3.6
DLD-1
11.6
14
MX-1
1.6
1.8
OVCAR-3
1.8
2.0
[0040] Mouse animal experiment results (the number of mice with abnormal conditions):
[0041]
[0042] Activity experiments showed that the semi-lethal dose (IC 50 ) values are all less than vinblastine, indicating that it has better anti-tumor activity than vinblastine; meanwhile, the mouse animal experiment results show that t...
Embodiment 3
[0044] Obtaining of compound A2
[0045] 6'-N oxidation-epoxy vinblastine (6'-N b -oxide-leurosine) at the 3-position for semi-synthetic modification, the original -COOCH at the 3-position 3 Replaced with -COOC 6 h 5 , whose structure is:
[0046]
[0047] Semi-lethal dose (IC 50 ) Experimental results:
[0048] IC 50 Value (μM / L)
[0049] cancer cell line
He La
14.6
16
A549
3.5
3.6
DLD-1
12.8
14
MX-1
1.8
1.8
OVCAR-3
1.8
2.0
[0050] Mouse animal experiment results (the number of mice with abnormal conditions):
[0051]
[0052]
[0053] Activity experiments showed that the semi-lethal dose (IC 50 ) and slightly vinblastine, indicating that its anti-tumor activity is equivalent to that of vinblastine; meanwhile, the mouse animal experiment results show that the amount of abnormal situation mice is not significantly d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com