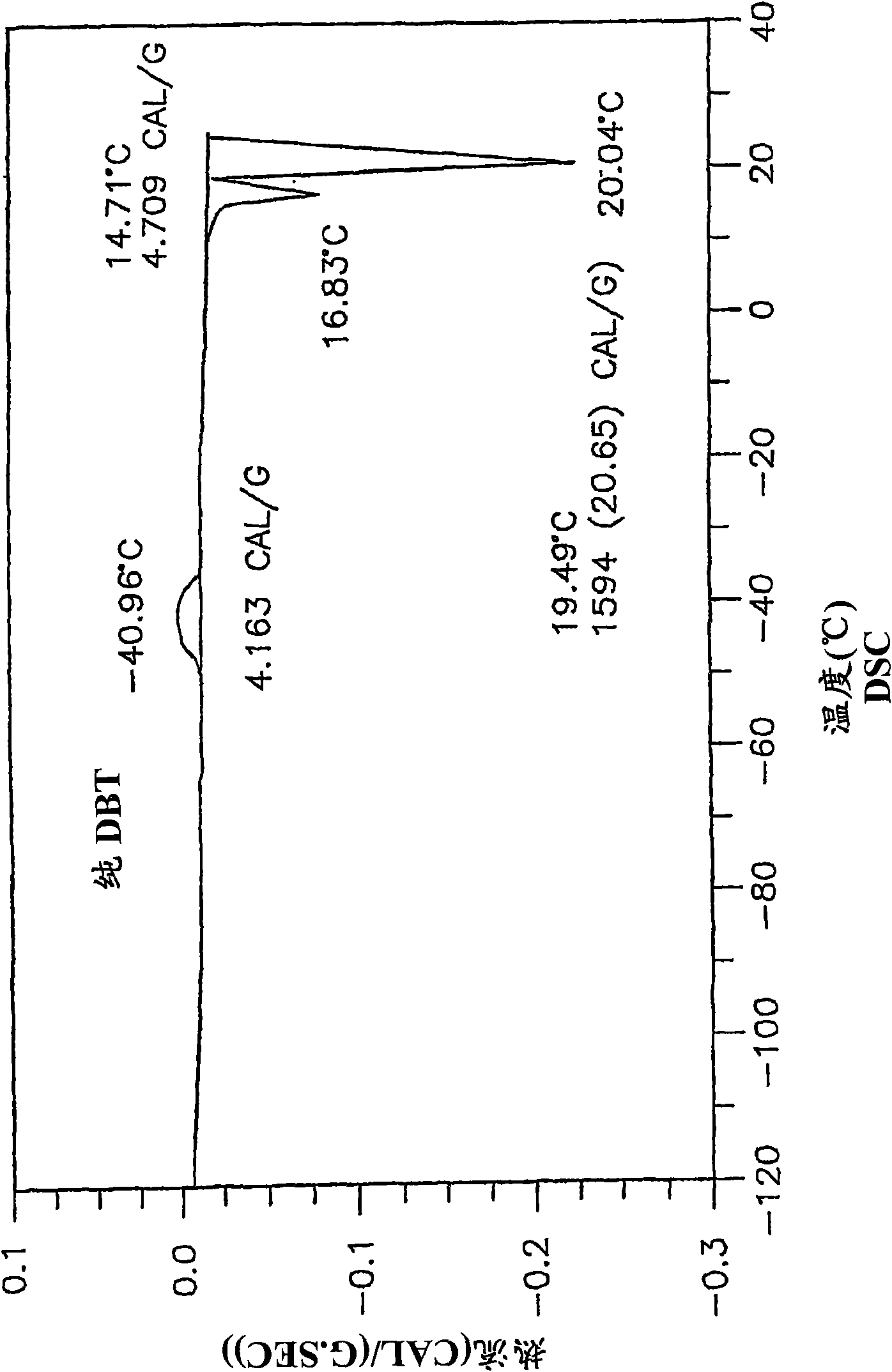
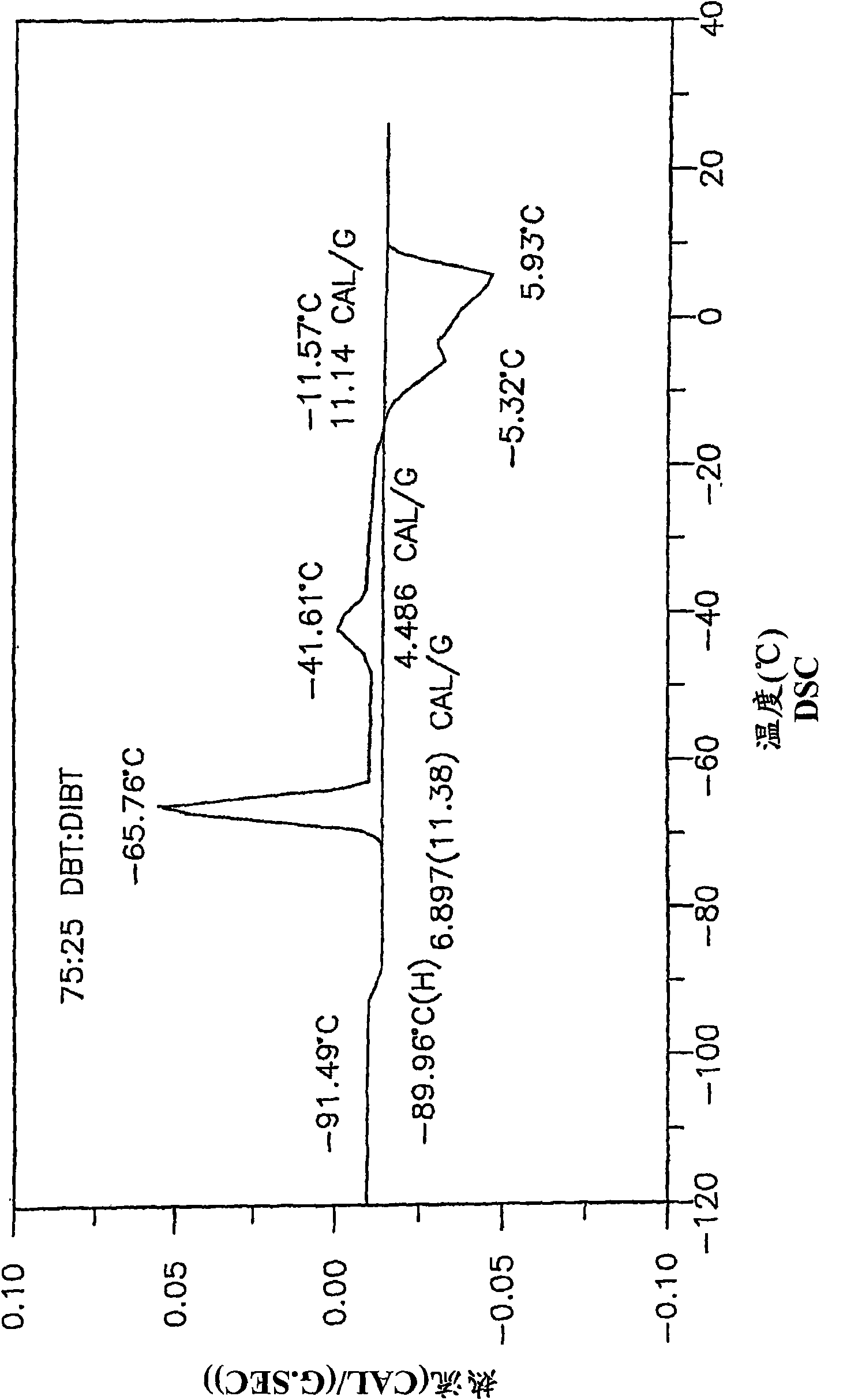
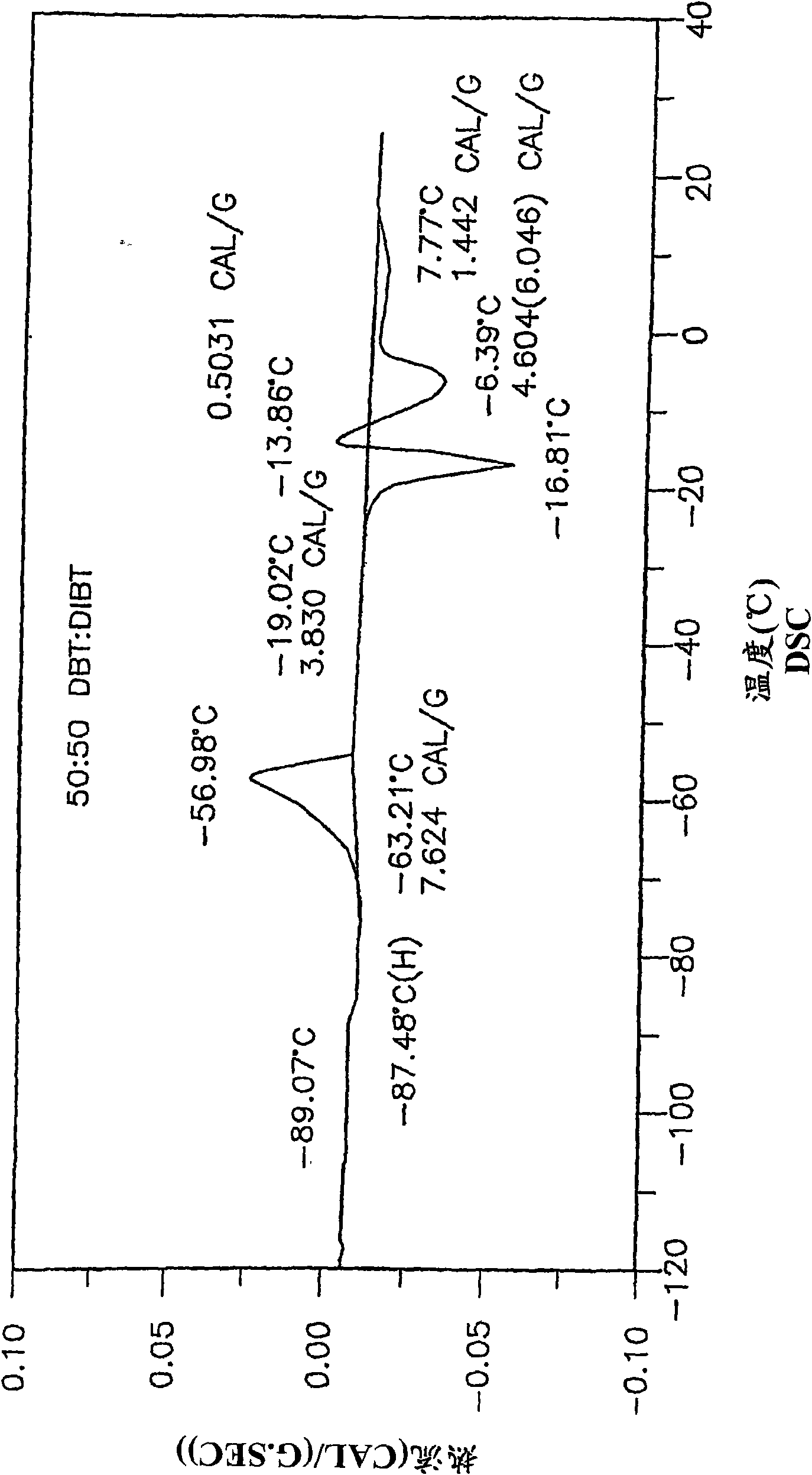
Low-melting mixtures of di-n-butyl and diisobutyl terephthalate
A technology of diisobutyl phthalate and di-n-butyl phthalate is applied in the field of mixtures of terephthalic acid diesters and can solve problems such as increasing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The reaction was carried out according to the general scheme illustrated on page 3 above, designed to give the product using a 50:50 mole percent mixture of n-butanol and isobutanol.
[0024] Add 436.9g (2.25mol, MW=194.19) dimethyl terephthalate, 207.5g (2.8mol, MW=74.12) n-butanol, 207.5g (2.8mol, MW=74.12) isobutanol and 223 ppm (0.19 g) titanium tetraisopropoxide (TIPT). The course of the reaction is summarized in Table 1 below:
[0025] Table 1
[0026] time
[0027] The stripped products are summarized in Table 2 below.
[0028] Table 2
[0029] stripping
[0030] After neutralization, drying, carbon treatment and finally filtration according to the general work-up procedure described above, the isolated product weighed 547.7 g. The theoretical amount of product is 626.28 g, so the isolated yield is 87.5%.
Embodiment 2
[0032] The reaction described in Example 1 was repeated to obtain the corresponding product from a 75:25 mole percent mixture of n-butanol and isobutanol. Add 233g (1.2mol, MW=194.19) dimethyl terephthalate, 200.1g (2.78mol, MW=74.12) n-butanol, 66.7g (0.9mol, MW=74.12) isobutanol and 220ppm to the reactor system (0.11 g) TIPT. The course of the reaction is summarized in Table 3 below:
[0033] table 3
[0034] time
[0035] The stripped product, as summarized in Example 1 above, was isolated as a clear liquid after neutralization, drying, carbon treatment and finally filtration.
Embodiment 3
[0037] The reaction described in Example 1 was repeated to obtain the corresponding product from a 25:75 mole percent mixture of n-butanol and isobutanol. Add 233g (1.2mol, MW=194.19) dimethyl terephthalate, 66.7g (0.9mol, MW=74.12) n-butanol, 200.1g (2.7mol, MW=74.12) isobutanol and 280ppm to the reactor system (0.14g) TIPT. The course of the reaction is summarized in Table 4 below:
[0038] Table 4:
[0039] time
Reaction time
(Hour)
Base
temperature °C
receive
temperature °C
receive
volume
(ml)
Notes
8:00
-
102
22
0
8:30
0.0
116
93
0
start reaction time; open access
@<66℃@20%
9:00
0.5
109
65
0
9:30
1.0
106
64
0
Lifter not working; shut down and replace
10:15
84
25
0
start heating
10:5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com