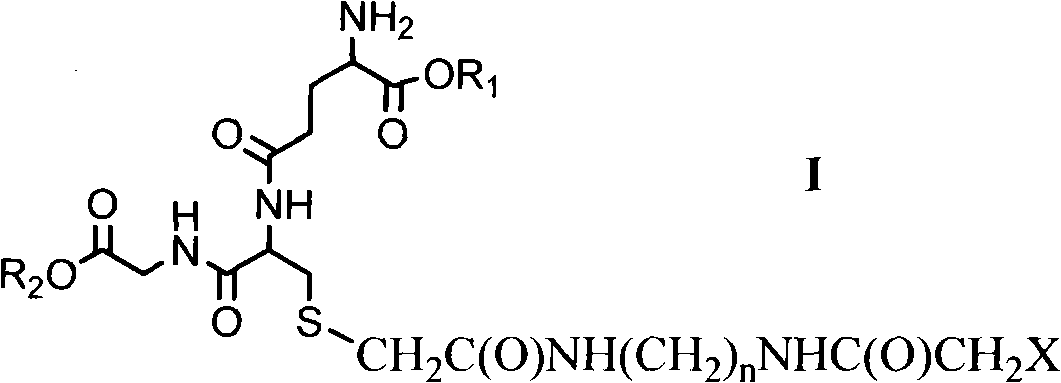
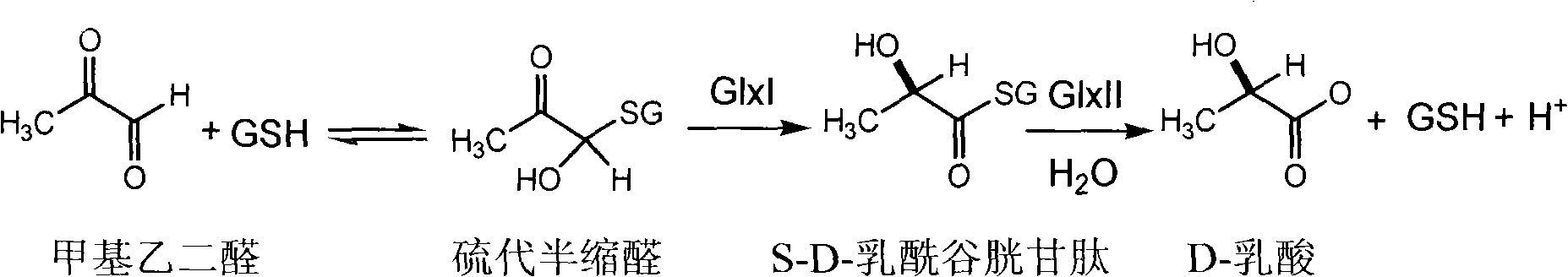
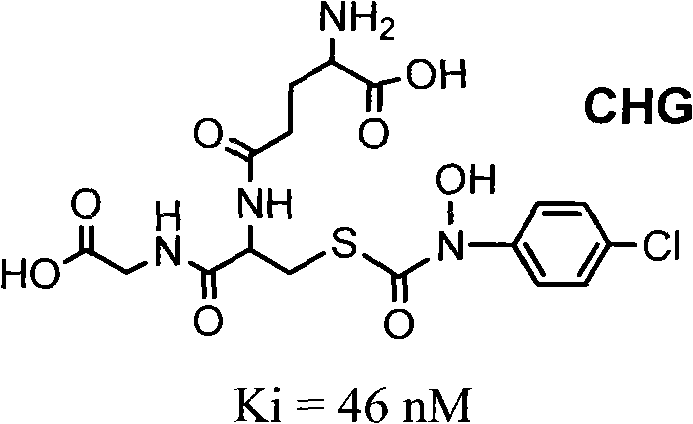
Glutathione derivative and anti-tumor medical application thereof
A glutathione and anti-tumor drug technology, applied in anti-tumor drugs, tripeptide components, drug combinations, etc., can solve the problem of low activity of GlxII
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] Synthetic Irreversible Inhibitors of GlxI
[0019] The synthetic pathways of S-(bromoacetamidoalanylmethyl)glutathione (BPSG) and S-(bromoacetamidobutanylmethyl)glutathione (BBSG) are as follows:
[0020]
[0021] Dibromoacetyl(1,3)propylenediamine
[0022] Put 50 milliliters of chloroform and 1,3-propanediamine (13mmol) in the flask of 100 milliliters, then put into ice bath and wait for cooling, slowly drop bromoacetyl chloride (1.03g, 26mmol) under stirring condition , Continue to stir for half an hour after dripping. The solvent was evaporated to dryness with a rotary evaporator under reduced pressure to obtain a light brown crude product. Yield: 90%. 1 H NMR 300MHz (CDCl 3 , TMS) δ3.80 (4H, s, -C(O)CH 2 Br), 3.25 (4H, t, J=6.25Hz, -NHCH 2 CH 2 -), 1.85 (2H, p, J=6.25Hz, -NHCH 2 CH 2 -).ESI MS: (M(Br 79 )+H + ) 314.93. Dibromoacetyl (1,4) butanediamine
[0023] The synthetic pathway of this compound is roughly the same as that of dibromoacetyl (1,3) p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com