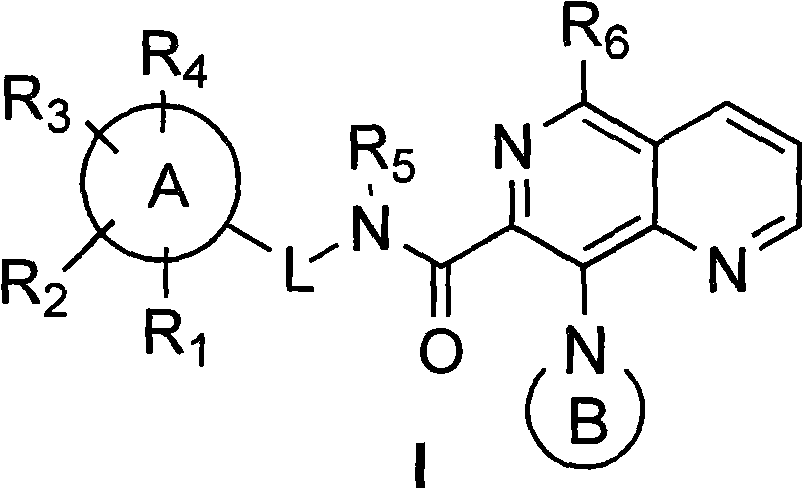
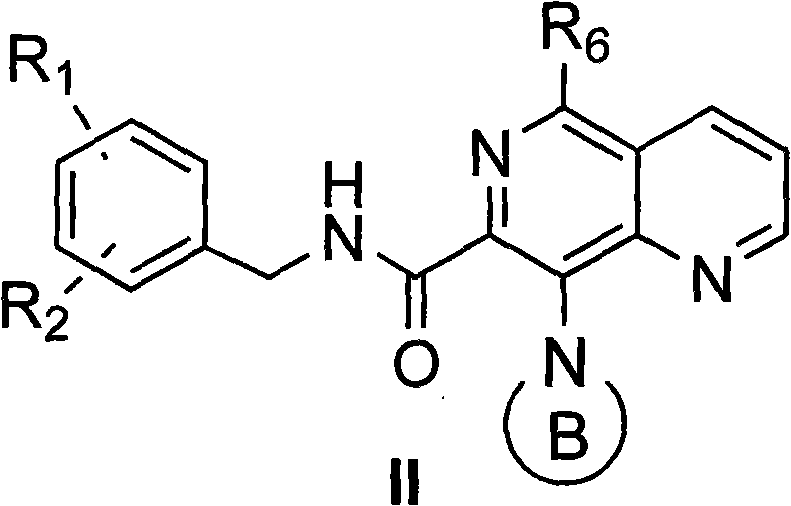
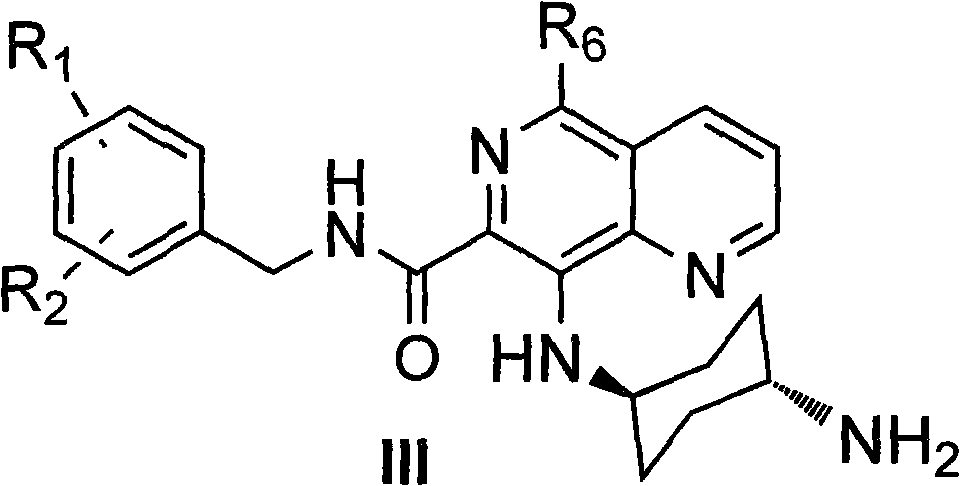
5,8-disubstituted-1,6-quinazoline-7-amidocarbonylation compound, preparing method, composite and application thereof
A phthalazine and disubstituted technology, which is applied in drug combination, organic chemistry, pharmaceutical formulation, etc., can solve the problem of blocking the growth of tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0064] Anti-tumor activity test:
[0065] Typical compounds in the present invention and their targets against various tumor cells (MDA-MB-435, Hct116p53- / -, Hct116p53+ / +, Mcf-7, NIH 189, SkBr-3, LnCap, LnHer, HT 29, HEY) The semi-growth inhibitory activity data (IC 50 ) were measured under standard conditions.
[0066] Example: growth inhibition experiments of compounds on human breast cancer cells MDA-MB-435 and SKBr-3.
[0067] (1) Production methods and sources of main reagents
[0068] RPMII640 was purchased from Gibco; sulforhodamine B was purchased from Sigma; trichloroacetic acid (TCA), acetic acid (HAC) and Tris base unbuffer were all analytically pure.
[0069] (2) Experimental procedure (sulfonylrhodamine B (SRB) protein staining method)
[0070] According to the growth rate of the cells, human breast cancer cells MDA-MB-435 and SKBr-3 in the logarithmic growth phase were inoculated in 96-well culture plates at 90 μl / well, grown adherently for 24 hours, and then...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com