Method for preparing and separating definitive endoderm
A technology for definitive endoderm and embryonic stem cells, which is applied in the field of preparation and isolation of definitive endoderm cells, and can solve problems such as the inability to better distinguish VE and DE
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
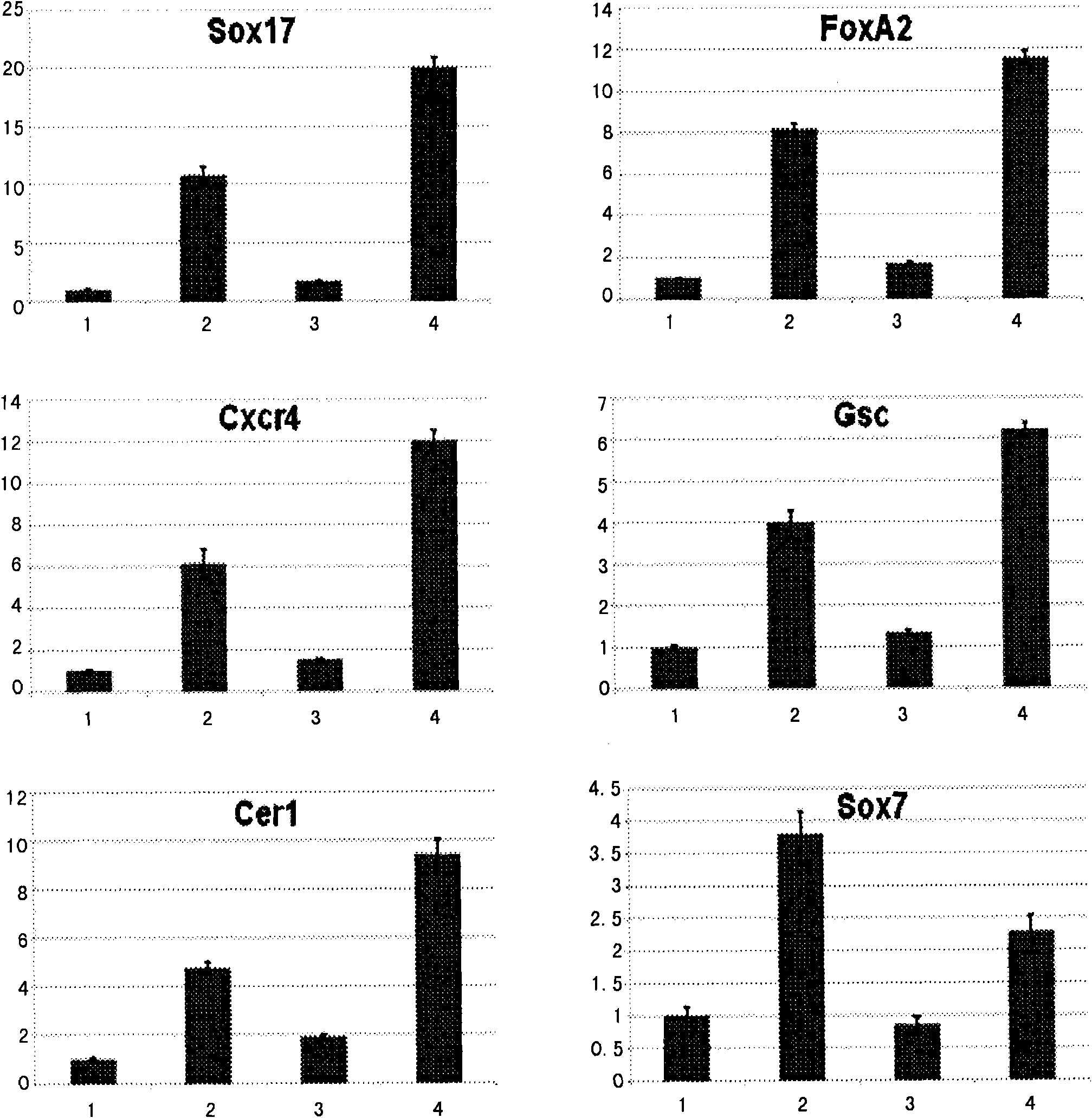
[0078] The present invention also includes the cell group containing definitive endoderm cells obtained by the aforementioned preparation method. In the cell population, the number of definitive endoderm cells accounts for more than 60% of the total number of cells, preferably more than 70% of the total number of cells; more preferably more than 80% of the total number of cells.
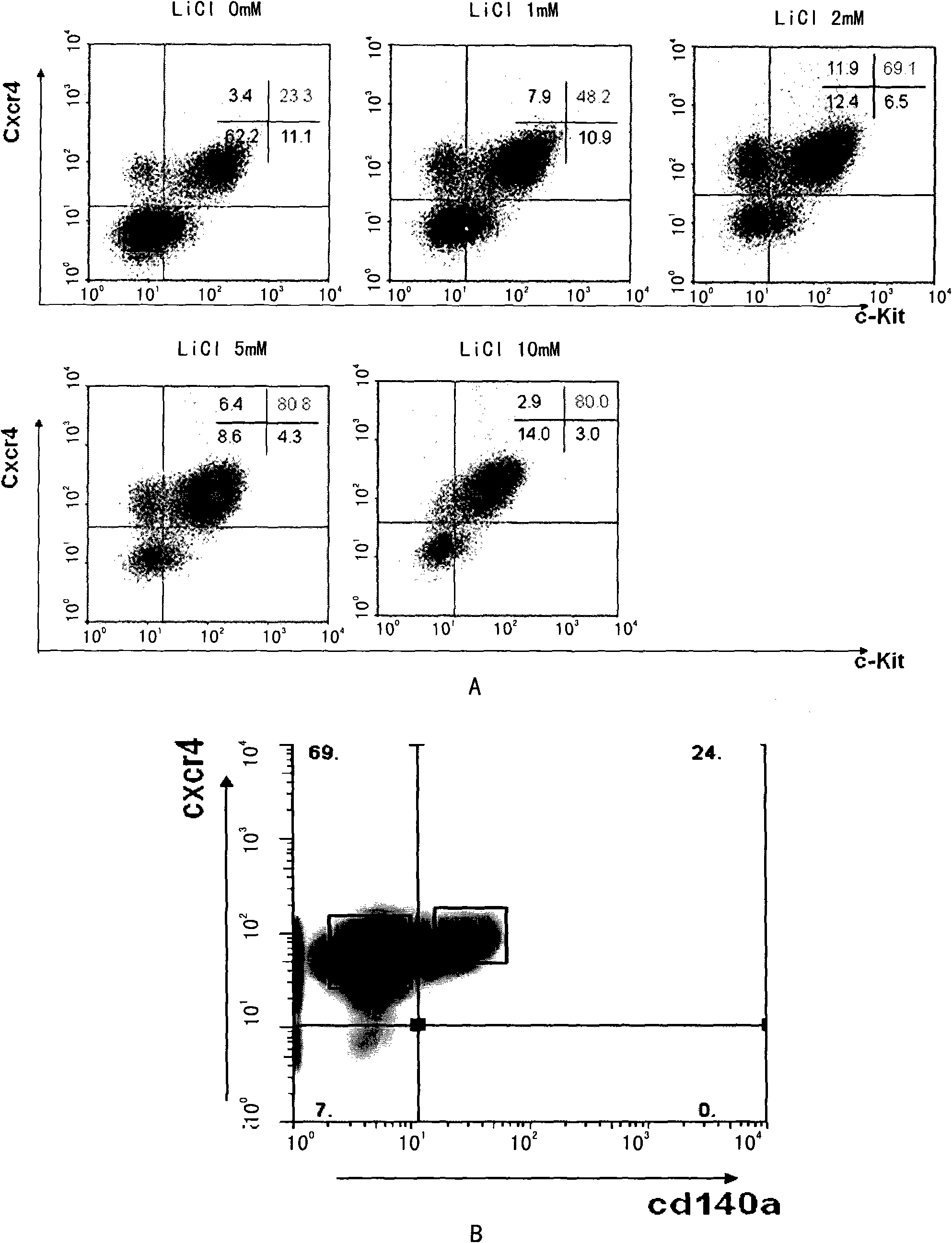
[0079] Using FACS and surface marker technology, it is possible to enrich definitive endoderm cells into Cxcr4+ / Cd140α- cell population or Cxcr4+ / EpCam+ cell population; use RT-PCR and other gene expression analysis techniques to prove the purity of the enriched endoderm cells Higher, less contamination from other germ layer cells.
[0080] Therefore, the present invention also includes cell populations with higher content of definitive endoderm cells separated by sorting techniques. Preferably, the cell population is a cell population positive for Cxcr4 molecules on the cell surface and positive fo...
Embodiment 1
[0106] Embodiment 1. Preparation of culture medium
[0107] 1. Preparation of serum-free medium
[0108] Prepare the mixture of IMDM and F12 according to the dosage shown in Table 1:
[0109] Table 1
[0110] IMDM
75% (volume ratio)
[0111] (available from Invitrogen; Cat# 12440-053, Gibco)
F12 mixture
(available from Invitrogen; Cat#: 11765-054, Gibco)
25% (volume ratio)
[0112] In the previously prepared mixture, add the ingredients of the formula in Table 2:
[0113] Table 2
[0114] N2
(available from Invitrogen, Cat: 17502-048)
0.5% (volume ratio)
B27 (Vitamin A deficiency)
(available from Invitrogen, Cat: 12587-010)
1% (volume ratio)
BSA
0.5mg / ml
penicillin or streptomycin (Gibco)
1% (volume ratio)
Vitamin C (Vc)
0.5mM
3-Mercaptothioglycerol (MTG)
4.5×10 -4 M
[0115] 2. Low serum culture medium
[0116] In GME...
Embodiment 2
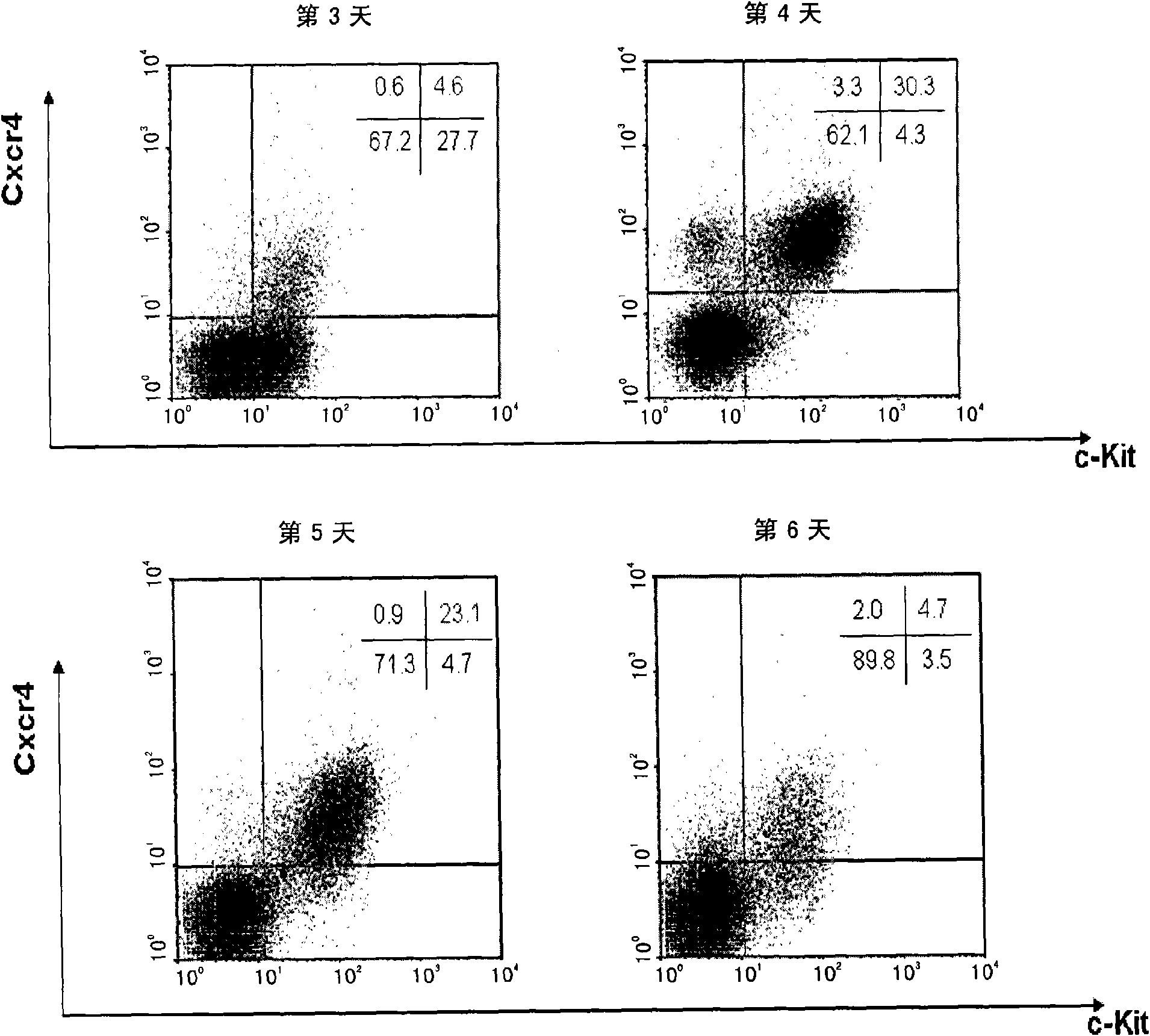
[0127] Example 2. Inducing mouse embryonic stem cells to differentiate into definitive endoderm cells and screening using Cxcr4+ / c-Kit+ markers
[0128] The method for inducing mouse embryonic stem cells to differentiate into definitive endoderm cells (DE) using the medium prepared above comprises:
[0129] 1) Mouse ES cells (purchased from ATCC, CRL-1821) were cultured in low serum to maintain an undifferentiated state;
[0130] 2) The mouse ES cells were digested with 0.25% Trypin-EDTA (Gibco), counted, according to 2×10 4 Individuals / ml with a total volume of 15ml were planted in a 10cm low-adherence bacterial culture dish, and the serum-free culture prepared above was used to culture under normal conditions for 48 hours to form embryoid bodies;
[0131] 3) Collect the embryoid bodies and continue to induce them with differentiation medium 6 for 48 hours.
[0132] 4) The induced embryoid bodies were digested into single cells, labeled with specific antibodies, and used at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com