Preparation method of 1, 7, 8- trifluoro-2- naphthol and derivative thereof
A technology of naphthol and dihydronaphthalene, applied in the field 1, can solve the problems of expensive palladium carbon catalyst and the danger of hydrogen, and achieve the effect of low cost, low price and simple operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
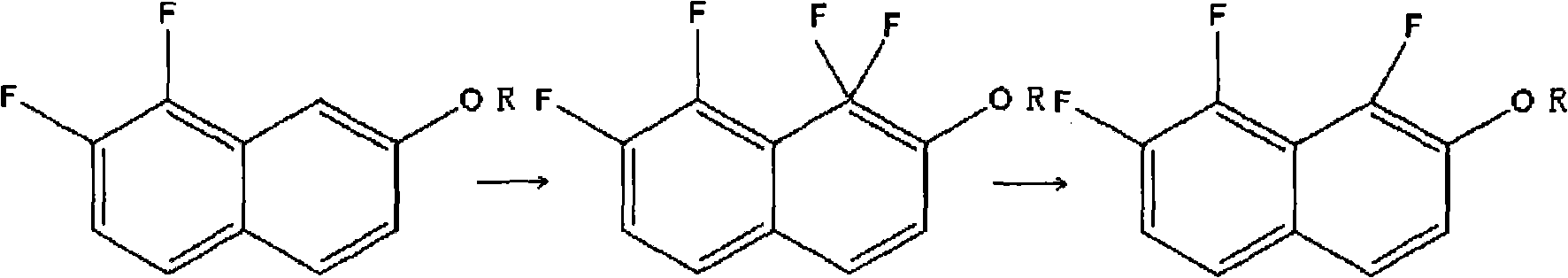
[0019] Embodiment 1: the preparation of 1,7,8-trifluoro-2-naphthol
[0020] 0.92g (0.005mol) of 7,8-difluoro-2-naphthol was dissolved in 35mL of acetonitrile in a 100mL three-necked flask, and heated to 50°C. Under continuous stirring, 5.35 g (0.0136 mol) of F-TEDA-BF4 was added, and the stirring was continued for 15 min. The solvent was rotovapped to obtain 0.54 g of a yellow solid, namely: 1,1,7,8-tetrafluoro-1,2-dihydronaphthalen-2-one.
[0021] Take 0.5 g of yellow solid, add 30 mL of acetonitrile, and heat to 50°C. Add 5mL of 0.95g (0.006mol) sodium formaldehyde sulfoxylate dihydrate (Sodium formaldehydesulfoxylate dihydrate) aqueous solution, and continue stirring at this temperature for 2.5h.
[0022] After filtering, the filtrate was separated into layers, and the organic phase was washed with 10 mL of 10% hydrochloric acid and washed with saturated brine three times, 5 mL each time. Dry over anhydrous sodium sulfate for 2 h, evaporate the solvent, and dry to obtain...
Embodiment 2
[0025] Embodiment 2: 1,7, the preparation of 8-trifluoro-2-naphthol
[0026] Take 0.92g (0.005mol) of 7,8-difluoro-2-naphthol and 35mL of acetonitrile into a 100mL three-necked flask, and raise the temperature to 50°C. Under constant stirring, 9.8 g (0.025 mol) of F-TEDA-BF4 was added. Stirring was continued for 20 min; a solid precipitated out. The solvent was rotovaped to obtain 0.48 g of a yellow solid. Namely: 1,1,7,8-tetrafluoro-1,2-dihydronaphthalene-2-one.
[0027] Take 1.0 g of 1,1,7,8-tetrafluoro-1,2-dihydronaphthalene-2-one, add 50 mL of acetonitrile, and heat to 55°C. Add 20mL of 3.8g (0.024mol) sodium formaldehyde sulfoxylate dihydrate aqueous solution. Stirring was continued at this temperature for 3h, resulting in a small amount of white solid.
[0028] After filtration, the filtrate was separated into layers, and the organic phase was washed with 30 mL of 10% hydrochloric acid, washed three times with saturated brine, dried over anhydrous sodium sulfate for ...
Embodiment 3
[0029] Embodiment 3: the preparation of 1,7,8-trifluoro-2-naphthol
[0030] Take 9.2g (0.05mol) of 7,8-difluoro-2-naphthol and 200mL of acetonitrile into a 500mL three-necked flask, and raise the temperature to 55°C. Under continuous stirring, 78.7 g (0.02 mol) of F-TEDA-BF4 was added. Stirring was continued for 30 minutes, and the reaction solution was filtered through celite.
[0031] The reaction solution obtained above in the fluorination step was heated to 50°C. Add 50mL of 7.3g (0.06mol) sodium formaldehyde sulfoxylate aqueous solution; continue stirring at this temperature for 2.5h, resulting in a small amount of white solid.
[0032] Filtration, the filtrate was separated, and the organic phase was taken, washed with 30 mL of 10% hydrochloric acid and then washed with saturated brine three times, dried over anhydrous sodium sulfate for 2 h, evaporated the solvent, and dried to obtain 7.3 g of a yellow solid. After recrystallization with toluene and petroleum ether [...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com