Inhibitors of akt/pkb with anti-tumor activity
A kit, technology of anticancer drugs, applied in the field of treating human or animal cancer or tumor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
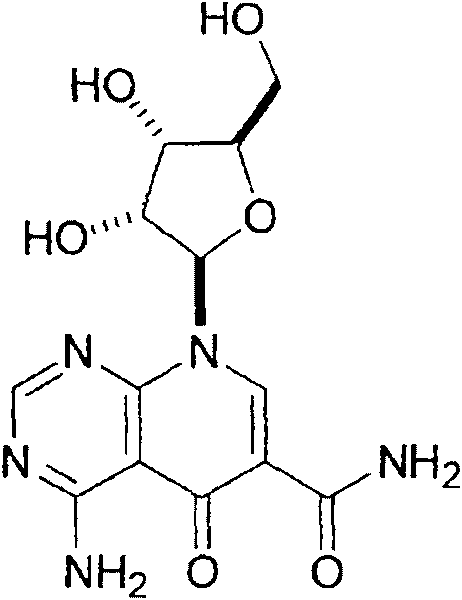
[0074] Example 1 - Small molecule Akt / PKB pathway by screening NCI Diversity Set Identification of Inhibitor-1, API-1
[0075] Due to the fact that aberrant activation of the Akt pathway occurs in almost 50% of all human malignancies and that inhibition of Akt induces cell growth arrest and apoptosis, both industry and academia are interested in the development of small molecules for anticancer drug development. Akt inhibitors have shown interest (Cheng et al., 2005; Granville et al., 2006). Although 12 Akt inhibitors have been reported, many of them lack antitumor activity in vivo. The lipid-based Akt inhibitor perifosine has been reported to be in Phase I and II studies (Van Ummersen et al., 2004; Bailey et al., 2006). However, the regulatory role of Akt was evaluated in none of these studies. A recent phase II study of perifoxine in pancreatic cancer was terminated due to unacceptable adverse effects during phase I (Marsh et al., 2007). However, there is a need to de...
Embodiment 2-A
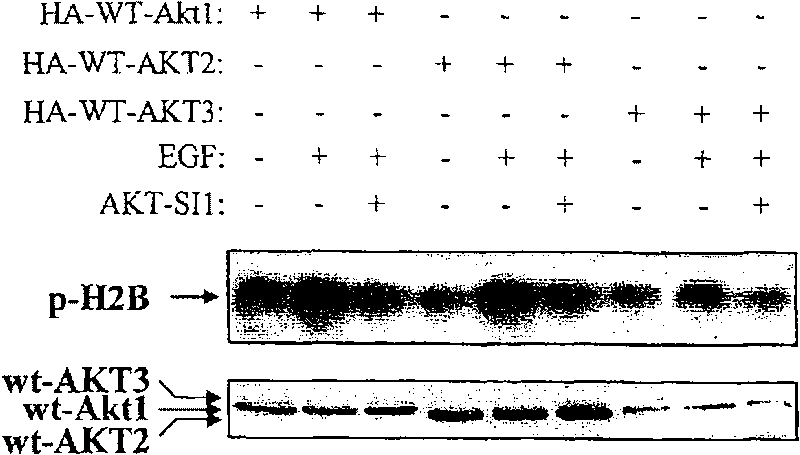
[0077] Example 2-API-1 does not inhibit the upstream activator of Akt
[0078] Akt is activated by extracellular stimuli and intracellular signaling molecules in a PI3K-dependent manner negatively regulated by PTEN. Activation of PI3K or mutation of PTEN activates PDK1, leading to induction of Akt kinase activity. Therefore, inhibition of Akt by API-1 may result from targeting upstream molecules of Akt, such as PI3K and PDK1. To this end, we next examined whether API-1 inhibits PI3K and / or PDK1. HEK293 cells were serum starved and then treated with API-1 or the PI3K inhibitor wortmannin for 1 hr before EGF stimulation. PI3K was immunoprecipitated with anti-p110α antibody. Immunoprecipitates were subjected to in vitro PI3K kinase assays using PI-4-P as substrate. as Figure 2A As shown in , EGF-induced PI3K activity was inhibited by wortmannin but not API-1. To evaluate the effect of API-1 on PDK1, we performed a PDK1 kinase assay (Upstate Biotechnology Inc.) in vitro u...
Embodiment 3
[0079] The high selectivity of embodiment 3-API-1 to Akt surpasses to AGC kinase member PKA, PKC and SGK and other signaling molecules ERK, JNK, p38 and STAT3
[0080] Akt belongs to the AGC (PKA / PKG / PKC) kinase family, which also includes PKA, PKC, serum- and glucocorticoid-inducible kinases (SGK), p90 ribosomal S6 kinase, p70 S6K , mitogen- and stress-activated protein kinases and PKC-related kinases. In the AGC kinase family, the protein structure of PKA, PKC and SGK is much closer to Akt kinase than other members. We therefore next examined the effect of API-1 on the enzymatic activity of these 3 kinases. In vitro PKA kinase assays and SGK kinase assays were performed with the indicated doses of API-1 or specific inhibitors pre-incubated with recombinant PKA or SGK proteins for 30 min, followed by addition of kemptide or Akt / SGK substrates in kinase buffer. Peptides and [γ- 32 P]ATP to initiate the kinase assay. In vitro kinase assays showed that the kinase activit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com