Process for the preparation of 17-(3-hydroxypropyl)-17-hydroxysteroids
A technology of hydroxysteroids and hydroxypropyl, which is applied in the production of steroids, organic chemistry, bulk chemicals, etc., and can solve the problems of pure products and yield reduction, propynol instability, difficult separation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] 6β, 7β; 15β, 16β-dimethylene-17α-(3-benzyloxypropynyl)androstane-3β, 5β, 17β-triol (Vb)
[0102] According to GWP1, 100 g (0.303 mol) of 3β, 5β-dihydroxy-6β, 7β; -Alcohol benzyl ether reaction.
[0103] The crude product was recrystallized from 700 ml of toluene. 133 g (0.279 mol) of 6β,7β;15β,16β-dimethylene-17α-(3-benzyloxypropyne)androst-3β,5β,17β-triol were obtained (92% yield of theory).
[0104] [α] D 20 =-70.1° (CHCl 3 , 12.15mg per 1ml solution, T=20°C, d=100mm).
[0105] 1 H-NMR (400MHz, CDCl 3 ): δ=0.37-0.42 (1H, m, H-30exo * ), 0.63(1H, td, J=9.0Hz and 5.1Hz, H-31endo), 0.78(1H, q, J=5.1Hz, H-31endo), 0.82-0.88(1H, m, H-6), 0.85 (3H, s, H-19), 0.91 (3H, s, H-18), 1.13 (1H, tt, J=8.4Hz and 4.3Hz, H-7), 1.15-1.27 (4H, m, H -30exo, H-1, H-9, H-11), 1.39-1.44 (1H, m, H-2α), 1.46-1.54 (3H, m, H-11, H-12β, H-15), 1.57 (1H, dt, J=13.6Hz and 2.9Hz, H-2β), 1.66-1.74 (3H, m, H-12α, H-16, H-8), 1.84 (1H, td, J=14.5Hz and 2.9Hz, H-1β), 1.96-2.01 (1H, m, H-4β)...
Embodiment 2
[0110] 15β,16β-methylene-17α-(3-benzyloxypropynyl)androst-6-ene-3β,5β,17β-triol
[0111] According to GWP1, 100 g (0.317 mol) of 3β, 5β-dihydroxy-15β, 16β-methylene-androst-6-en-17-one was mixed with 50.9 g (0.349 mol) of prop-1-yn-3 -Alcohol benzyl ether reaction.
[0112] The crude product was recrystallized from 700 ml of toluene. 134.5 g (0.291 mol) of 15β,16β-methylene-17α-(3-benzyloxypropynyl)androst-6-ene-3β,5β,17β-triol were obtained (92% theoretical yield) .
[0113] [α] D 20 =-120.3° (CHCl 3 , 12.15mg per 1ml solution, T=20℃, d=100mm)
[0114] 1 H-NMR (400MHz, CDCl 3 ): δ = 0.35-0.42 (1H, m, H-30exo), 0.95 (3H, s, H-18), 0.96 (3H, s, H-19), 1.14 (1H, ddd J = 6.8Hz, 3.7 Hz and 3.5Hz, H-30endo * ), 1.28-1.35 (1H, m, H-11β), 1.38-1.42 (1H, m, H-15), 1.45-1.51 (2H, m, H-1β, H-2), 1.50-1.60 (3H , m, H-12β, H-11α, H-9), 1.60-1.65 (1H, m, H-2), 1.67-1.73 (2H, m, H-16, H-12α), 1.83-1.89 ( 1H, m, H-1α), 1.88-1.97 (3H, m, both H-4, H-14), 2.15-2.19 (1H, m, H-8), 2....
Embodiment 3
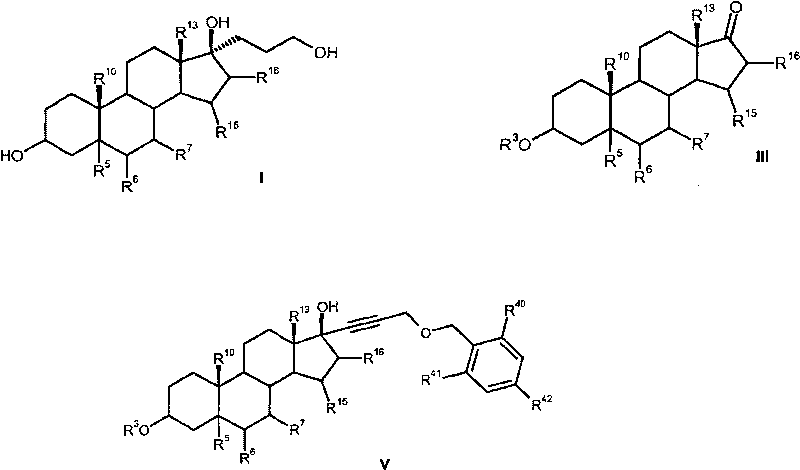
[0123] 6β, 7β; 15β, 16β-dimethylene-17α-(3-hydroxypropyl)androstane-3β, 5β, 17β-triol (Ia):
[0124] 100 g (0.210 mol) of 6β, 7β; 15β, 16β-dimethylene-17α-(3-benzyloxypropynyl)androstane-3β, 5β, 17β-triol was reacted according to GWP2 or GWP3, 81.1 g (0.208 mol) of 6β,7β;15β,16β-dimethylene-17α-(3-hydroxypropyl)androstane-3β,5β,17β-triol were obtained (99% yield of theory).
[0125] MS(CI): m / e=389(M-H) + , 373 (M+H-H 2 O) + , 355 (373-H 2 O), 337(355-H 2 O), 319(337-H 2 O).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com