Method for preparing Mn(OH)2 by circularly using ammonia water
A technology of ammonia water and concentration, applied in the direction of manganese oxide/manganese hydroxide, etc., can solve the problems of less research, easy oxidation, instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
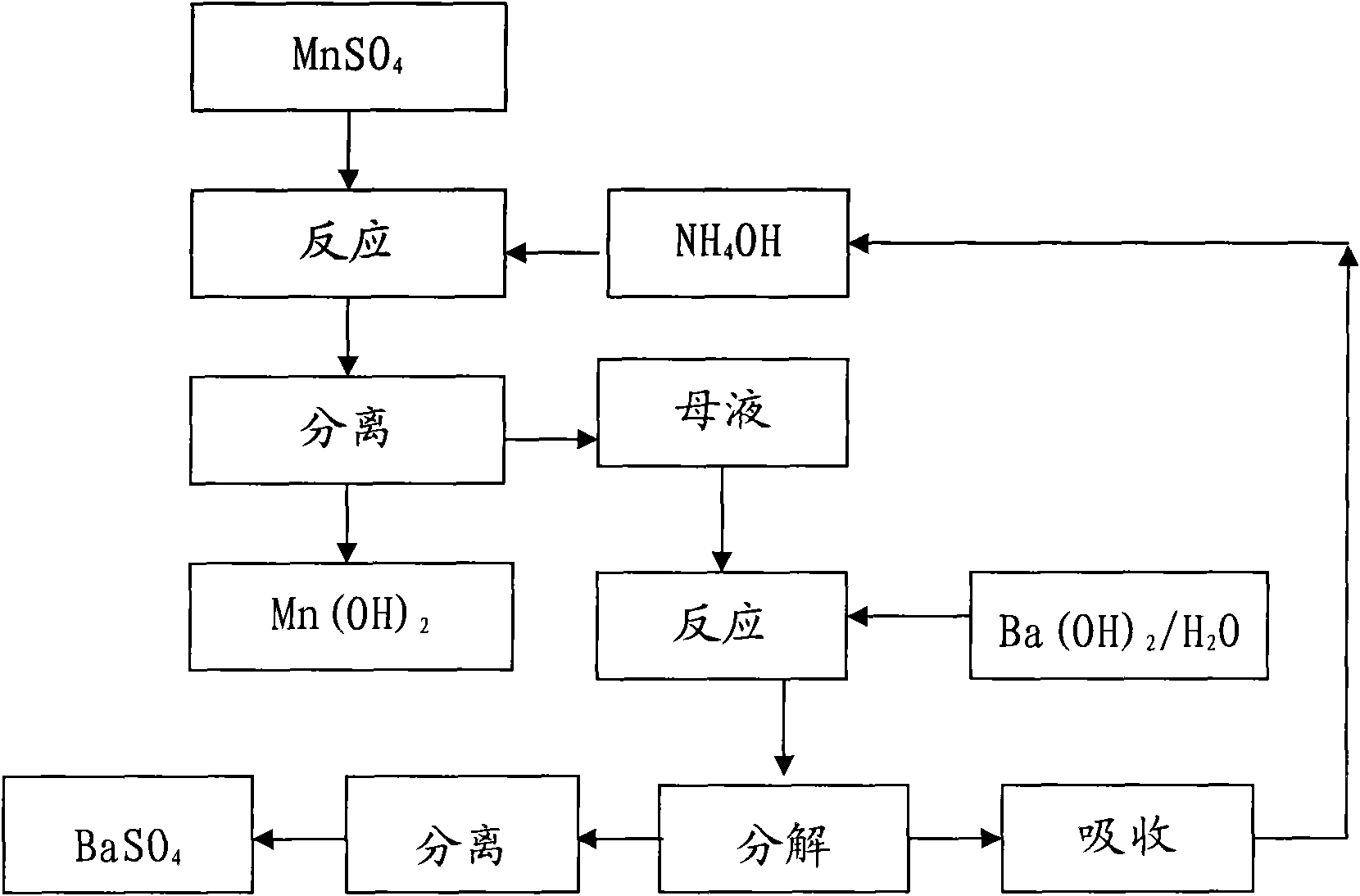
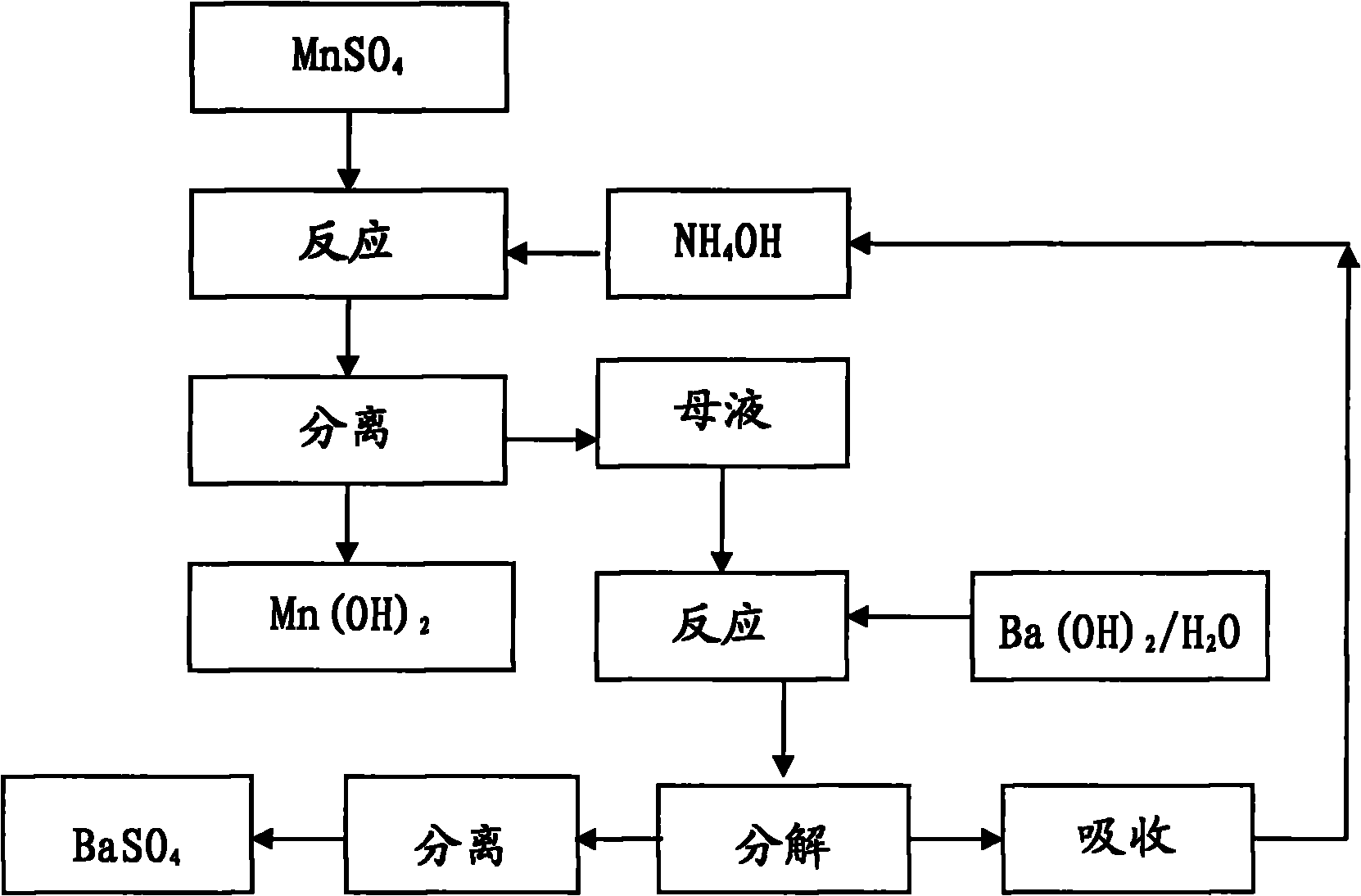
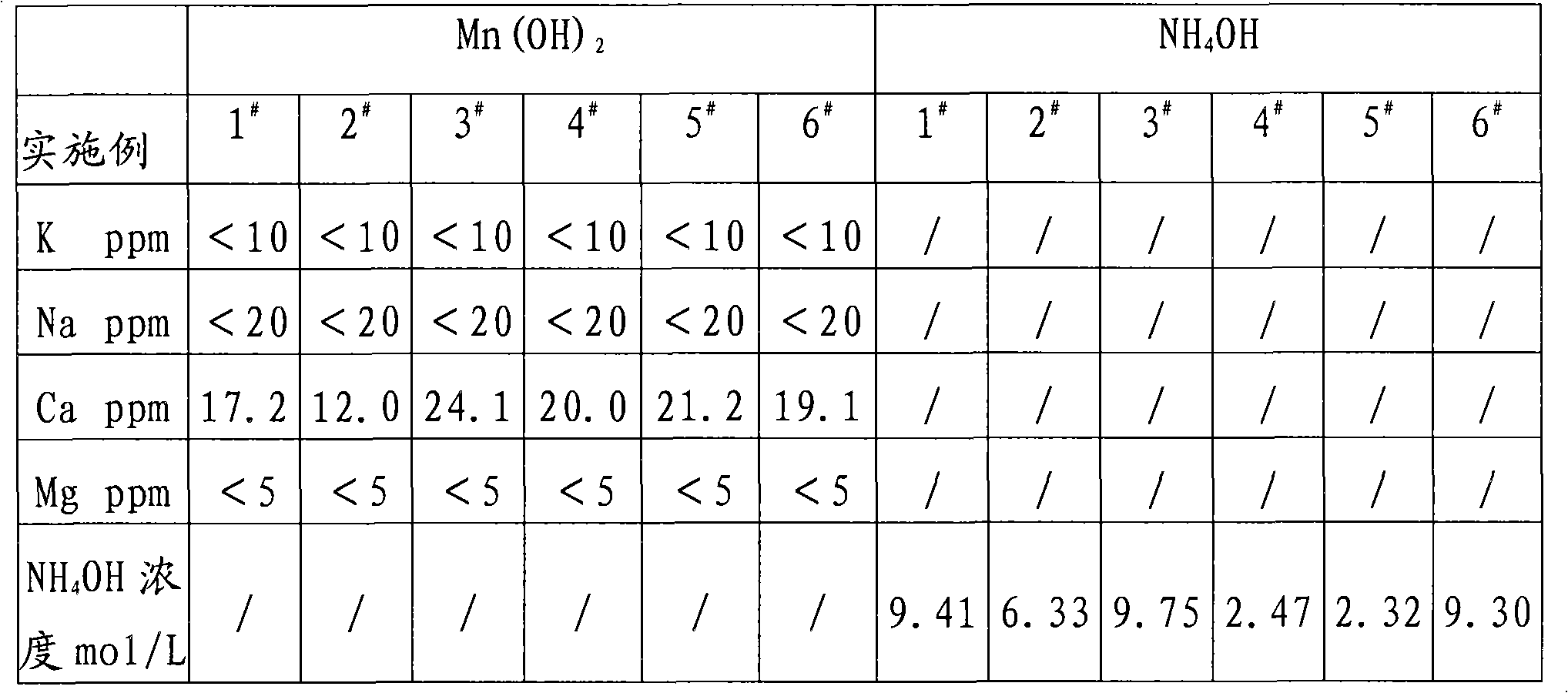
[0018] Manganese oxide powder and Sr(HS) 2 The solution is reacted, and the MnSO obtained after washing, separating, and sulfuric acid compounding 4 The solution was precision filtered with a 0.5 μm filter membrane, and the measured concentration was 450g / L. Measure 3000mL into a 5000mL beaker, and add 10mol / L NH 4 OH 1790ml, measured solution pH = 8.0, maintained stirring for 30 minutes and suction filtered, rinsed the filter cake with a small amount of deionized water, combined the filtrate for recycling regeneration treatment, Mn(OH) 2 Dry in a vacuum oven under nitrogen protection at 60°C for 16 hours to obtain Mn(OH) 2 sample 1 # .
[0019] Take the above filtrate - the concentration is 1.76mol / L (NH4) 2 SO 4 Put 2500ml of the solution in a 5000mL three-neck flask, connect the second stage to a bubbling absorption device equipped with deionized water in a cold water bath, turn on the vacuum pump to form a slight negative pressure, and slowly add 1470mL of 3mol / L Ba(O...
Embodiment 2
[0021] Manganese oxide powder and Sr(HS) 2 The solution is reacted, and the MnSO obtained after washing, separating, and sulfuric acid compounding 4 The solution was precision filtered with a 0.5 μm filter membrane, and the measured concentration was 200g / L. Measure 2000mL into a 5000mL beaker, and add 2mol / L NH 4 OH 2650ml, measure the solution pH=7.5, keep stirring for 30 minutes and then suction filter, rinse the filter cake with a small amount of deionized water, combine the filtrate for recycling regeneration treatment, Mn(OH) 2 Dry in a vacuum oven under nitrogen protection at 60°C for 16 hours to obtain Mn(OH) 2 sample 2 # .
[0022] Get above-mentioned filtrate---concentration is 0.53mol / L (NH 4 ) 2 SO 4 Put 3000ml of the solution in a 5000mL three-neck flask, connect the second stage to a bubbling absorption device equipped with deionized water in a cold water bath, turn on the vacuum pump to form a slight negative pressure, and slowly add 795mL of 2mol / L Ba(OH)...
Embodiment 3
[0024] Commercially available feed grade MnSO 4 ·H 2 O was added to deionized water to prepare 450g / LMnSO 4 Solution, add MnS made by Guizhou Hongxing Development Co., Ltd. under stirring to remove impurities, and filter it with a 0.5 μm filter membrane to measure MnSO 4 447.5g / L, put 3000ml in a 5000mL beaker, add 10mol / L NH 4 OH1780ml, measure the solution pH=8.0, maintain stirring for 30 minutes, then suction filter, rinse the filter cake with a small amount of deionized water, and combine the filtrates for recycling regeneration treatment, Mn(OH) 2 Dry in a vacuum oven under nitrogen protection at 60°C for 16 hours to obtain Mn(OH) 2 sample 3 # .
[0025] Get the above-mentioned filtrate—concentration is 1.74mol / L (NH4) 2 SO 4 Put 3000ml of the solution in a 5000mL three-necked flask, connect the second stage to a bubbling absorption device equipped with deionized water in a cold water bath, turn on the vacuum pump to form a slight negative pressure, and slowly add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com