Chiral phosphoramides, chiral N-phosphonimines and methods for forming the same
A phosphorimide and phosphoramide technology, applied in the field of design and synthesis of novel chiral imine reagents, can solve the problems of expensive raw materials, odorous disulfides, harmful substances, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
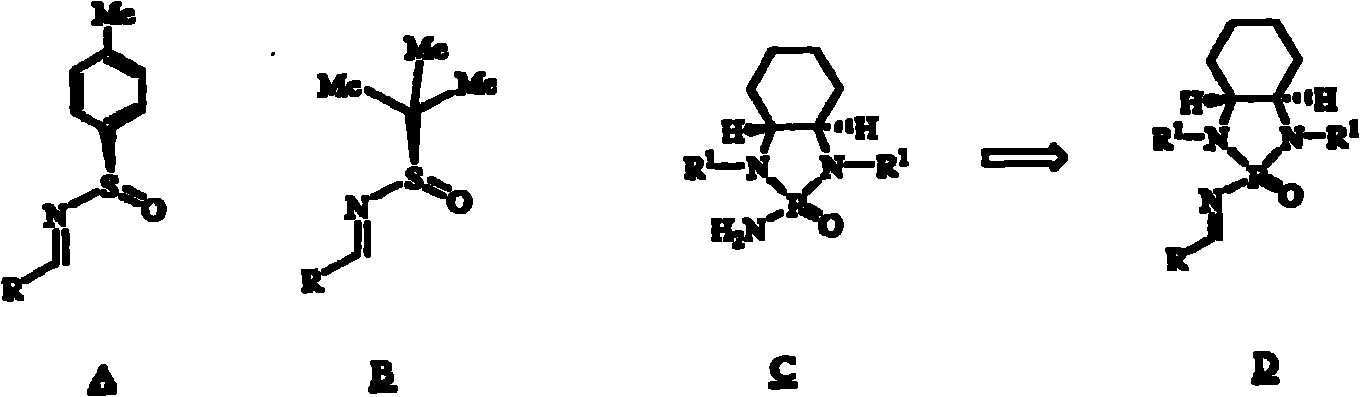
[0053] Synthesis of Chiral Phosphoramides Linked to Free Amino Groups
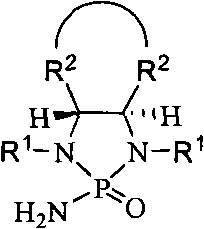
[0054] There are four steps in the synthesis of the chiral phosphoramide, and the intermediate products in these steps can be directly subjected to the next reaction without purification. Similar to the steps of the synthesis of N-alkyl phosphoramides, protected 1,2-diamines or amino alcohols can be used as raw materials for the preparation of the chiral phosphoramides (S.E.Denmark, Nature, 2006,443,40-41; S.E. Denmark, R.A. Stavenger, J.Am.Chem.Soc.2000, 122, 8837-8847; S.E.Denmark, Y.-P.Su, Y.Nishigaichi, D.M.Coe, K.T.Wong, S.B.D.Winter, J.Y.Choi, J.Org . Chem. 1999, 64, 1958-1967).
[0055] A.N, the synthesis of N'-diprimary alkyl-1,2-cyclohexanediamine (formula (V))
[0056] 1) Synthesis of (1R, 2R)-N, N'dibenzyl-1,2-cyclohexanediamine 2
[0057] In a 250 mL three-neck round bottom flask equipped with a calcium chloride drying tube, add (1R,2R)-(-)-1,2-cyclohexanediamine 1 (2.283 g, 20.0 mmol) and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com