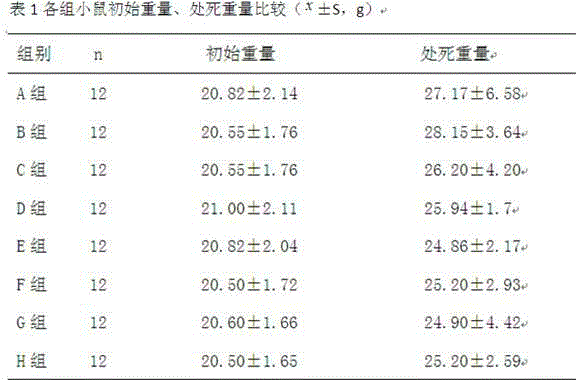
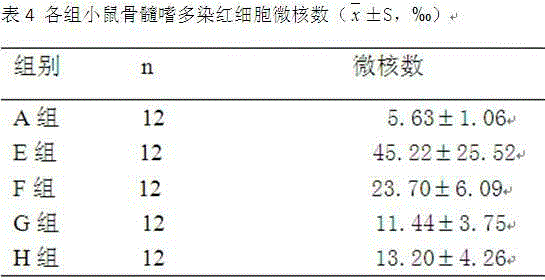
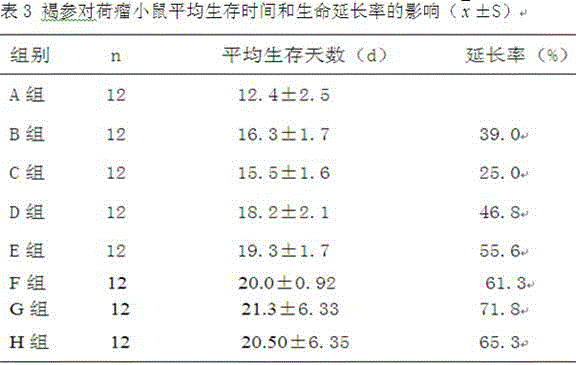
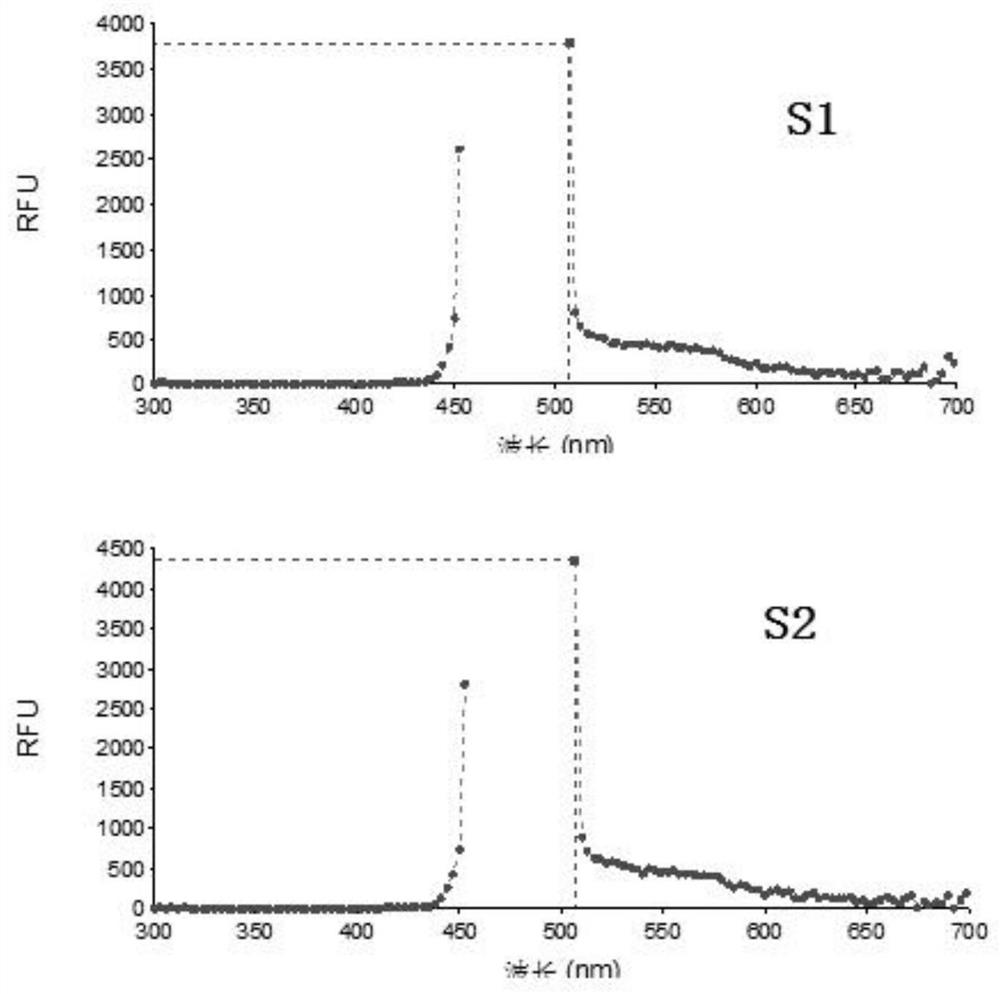
Patents
Literature
132 results about "Phosphoramides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
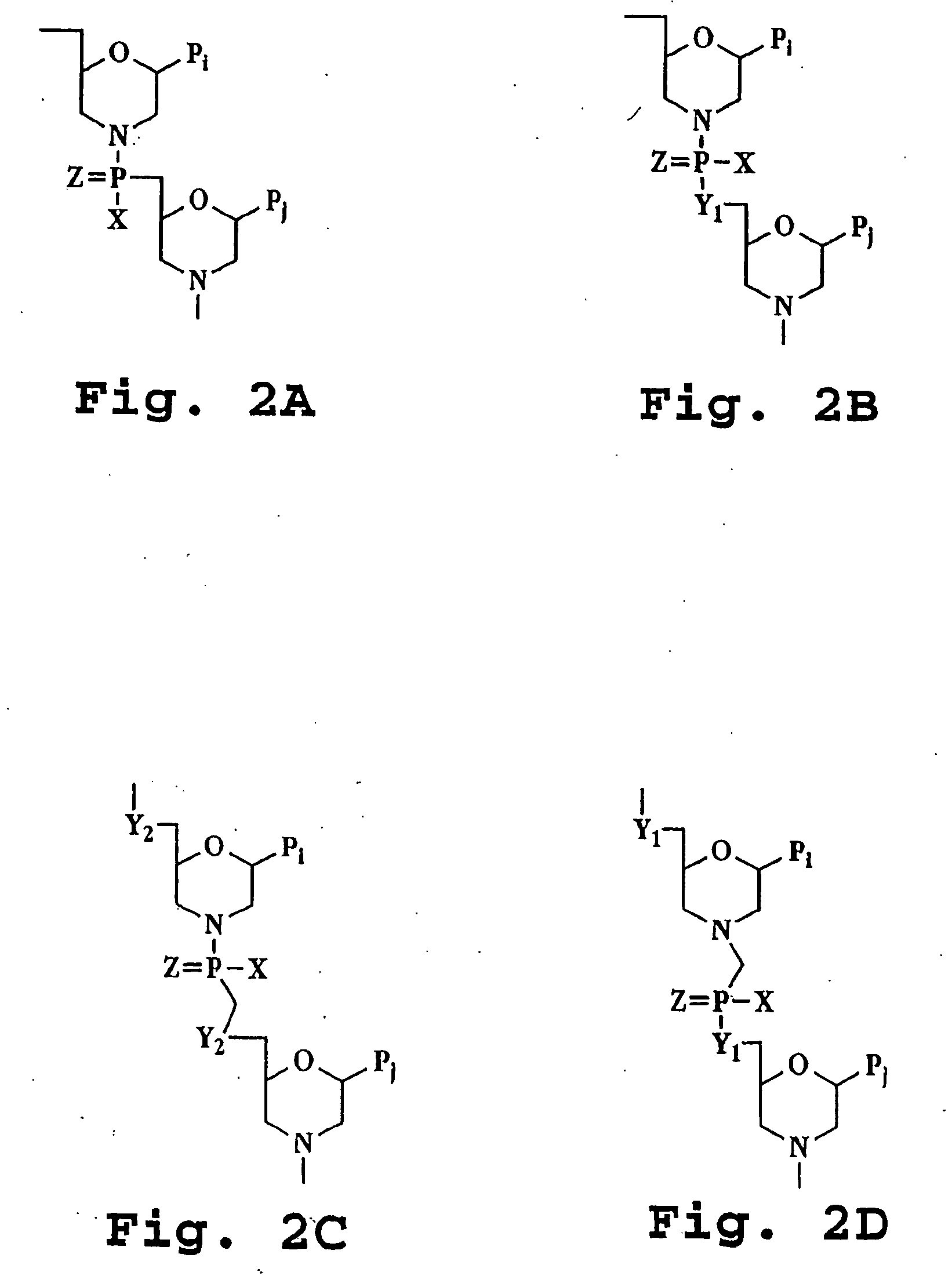
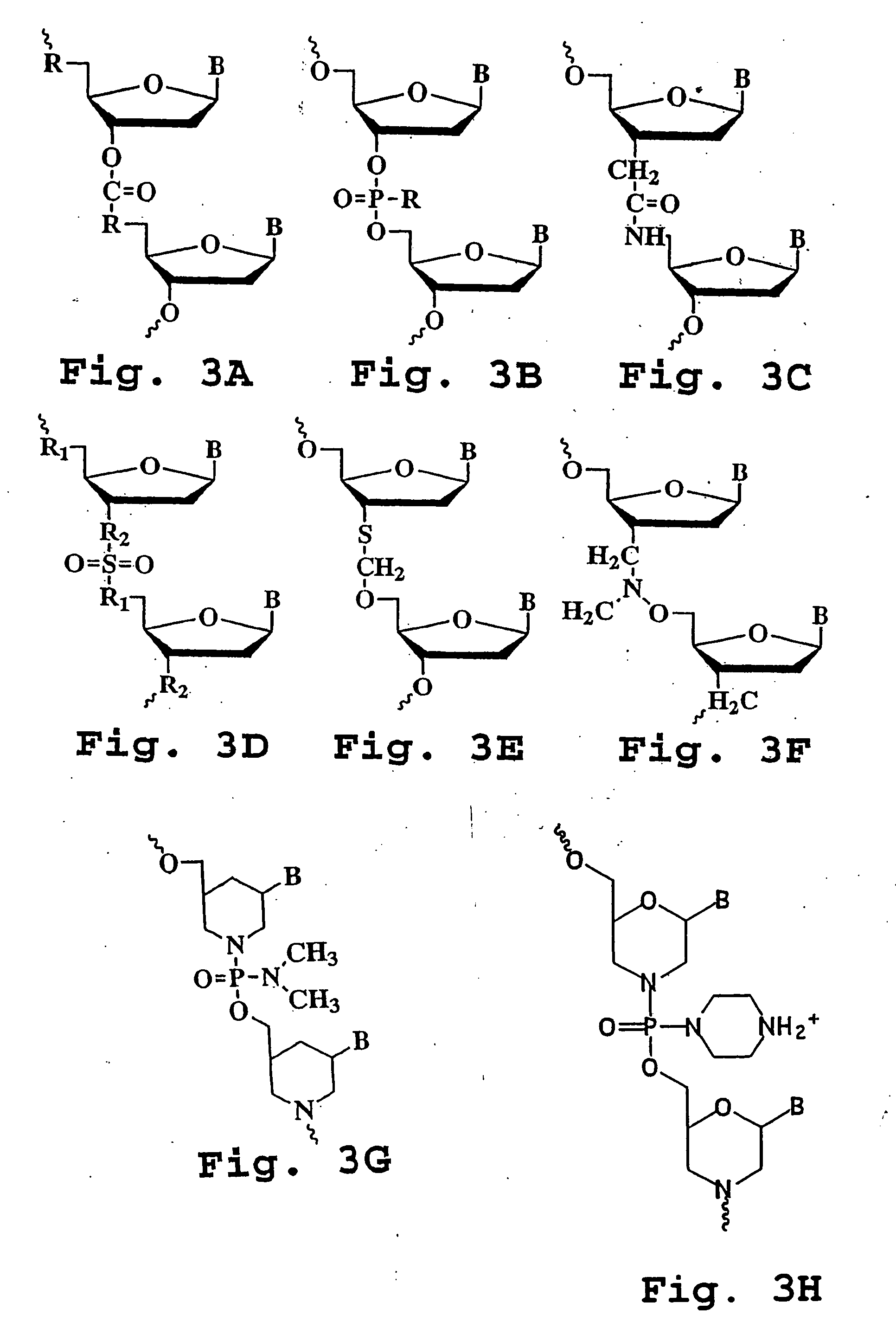
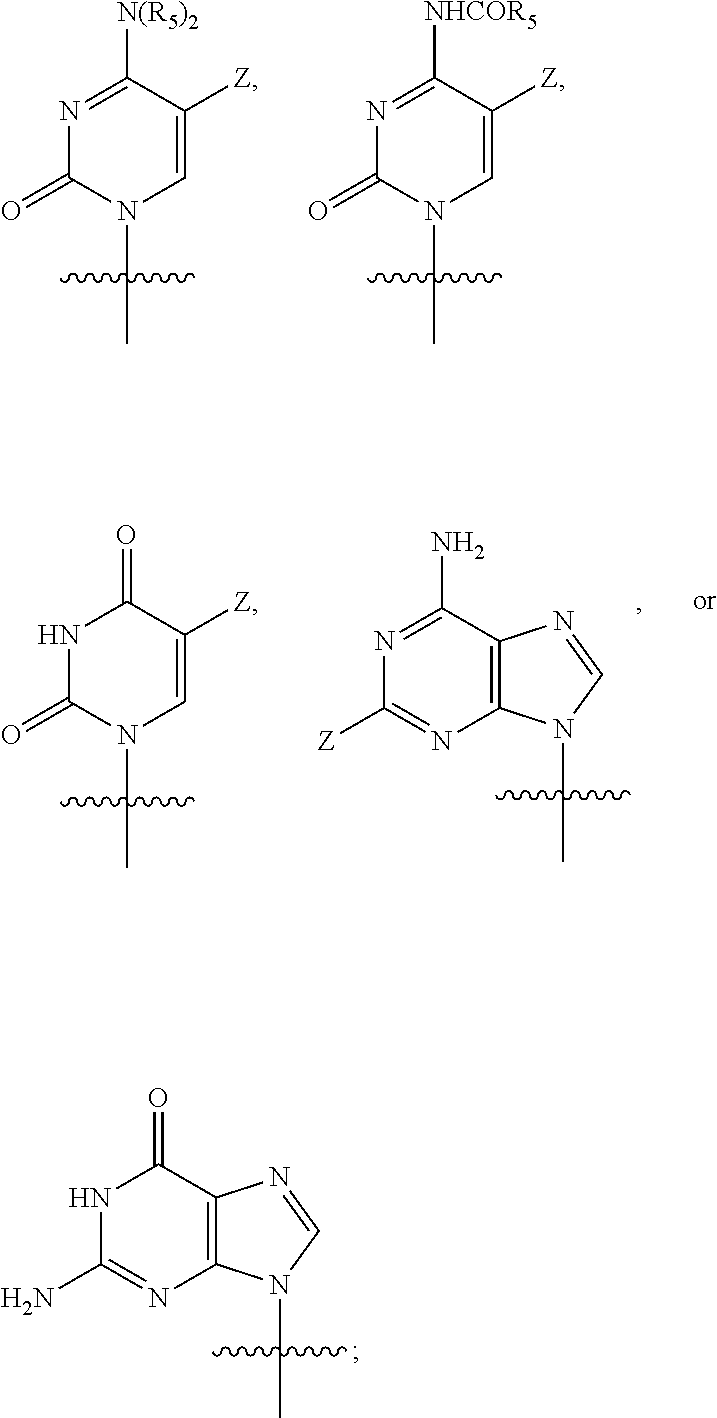
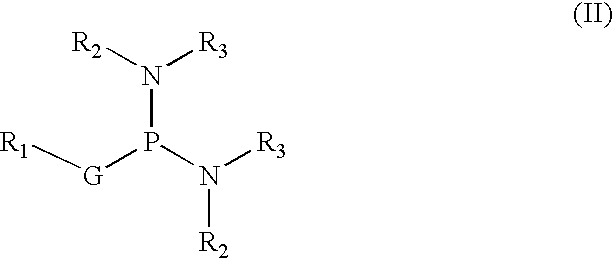
Amide derivatives of phosphoric acid such as compounds that include the phosphoric triamide (P(=O)(N)(N)(N)) structure.
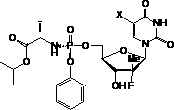
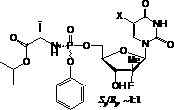
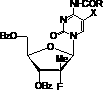
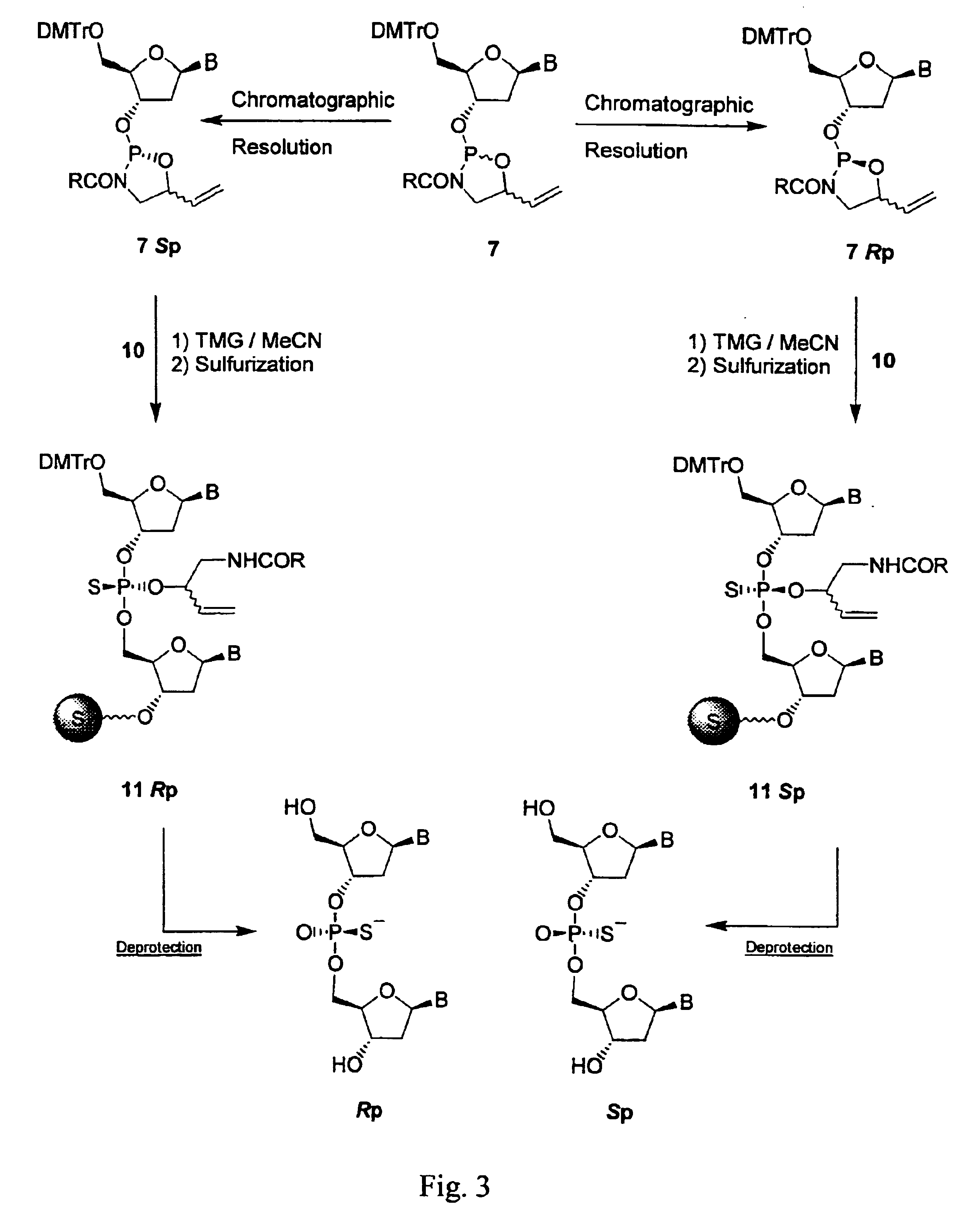
Nucleoside phosphamide prodrug as well as preparation method and application of nucleoside phosphamide prodrug
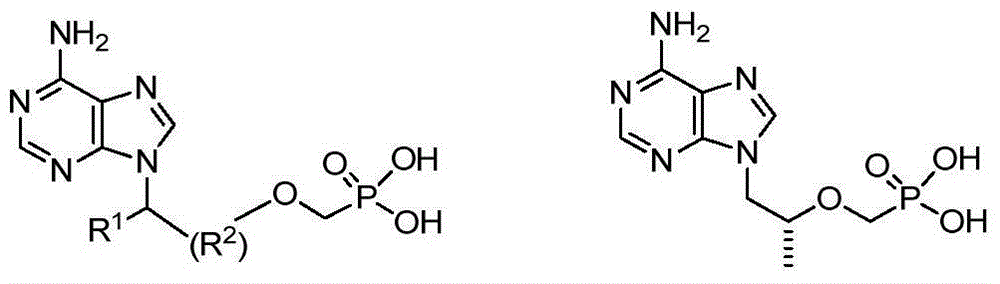
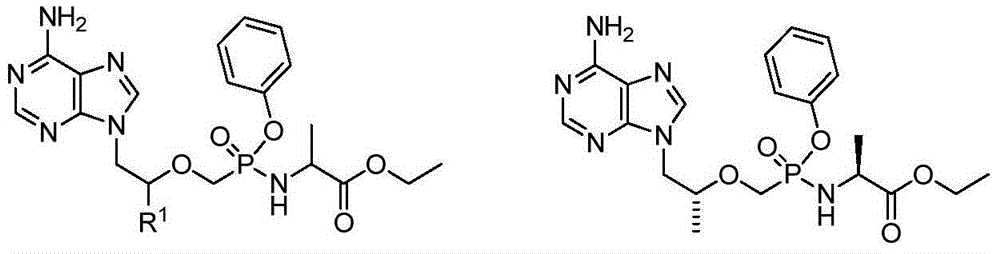
The invention relates to a nucleoside phosphamide prodrug as well as a preparation method and application of the nucleoside phosphamide prodrug. The nucleoside phosphamide prodrug is selected from any one of a compound I and a compound II, wherein in the formulas of the compound I and the compound II, X is selected from any one of F, Cl, Br and I. Compared with GS7977andGS7851, The compound I or II disclosed by the invention has more excellent resistance to hepatitis C virus, wherein the formulas I and II are respectively as shown in specifications.
Owner:ANHUI BIOCHEM UNITED PHARMA CO LTD
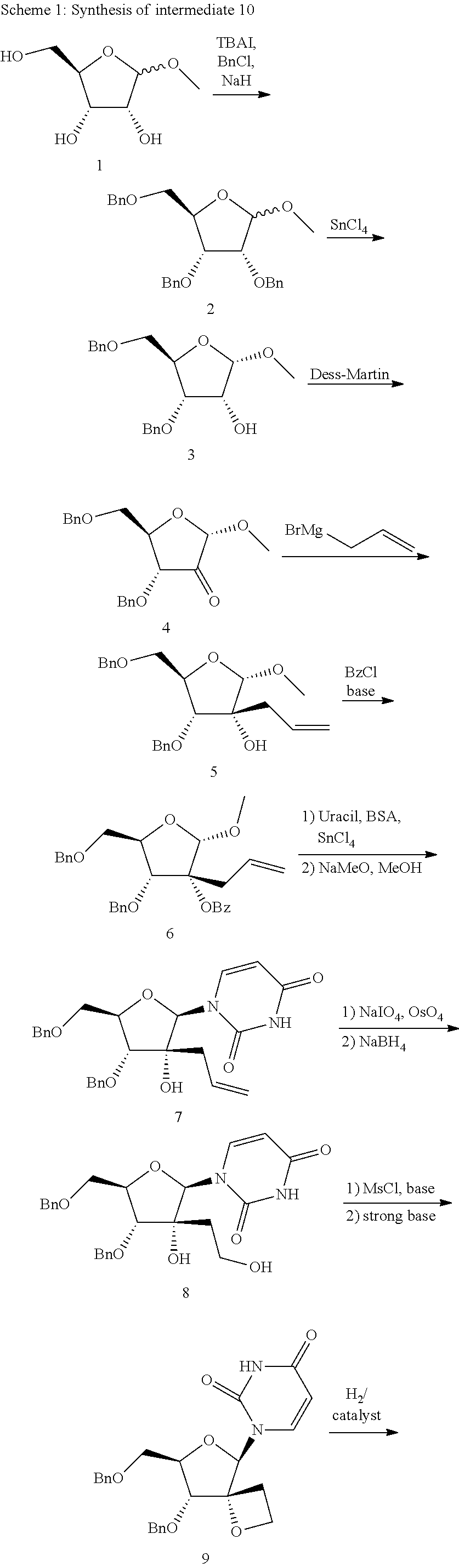
Uracyl Spirooxetane Nucleoside Phosphoramidates
This invention relates to a stereochemically pure uracyl spirooxetane nucleoside phosphoramidate useful in the treatment of patients infected with the hepatitis C virus (HCV).
Owner:TIBOTEC NV +2
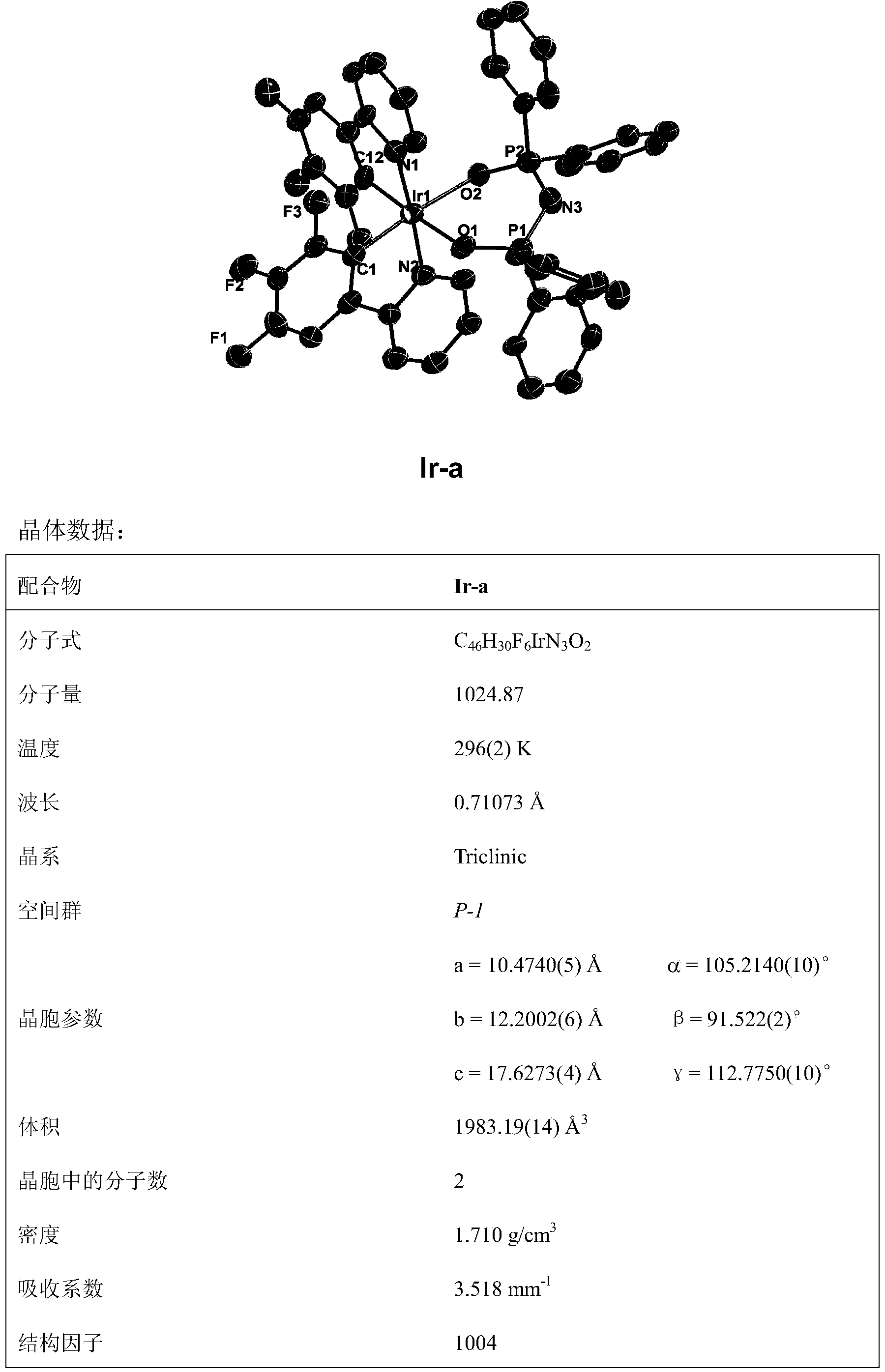
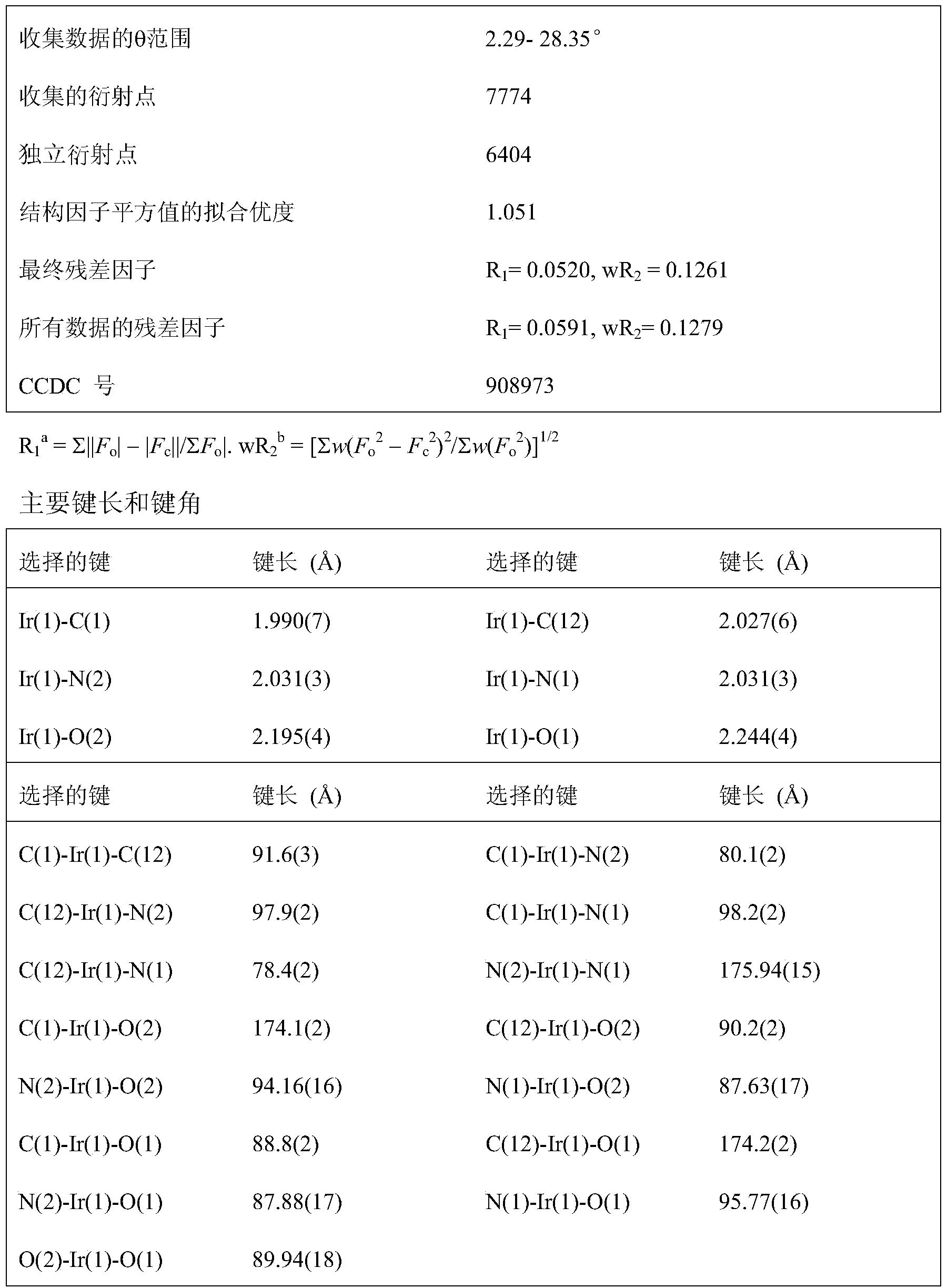
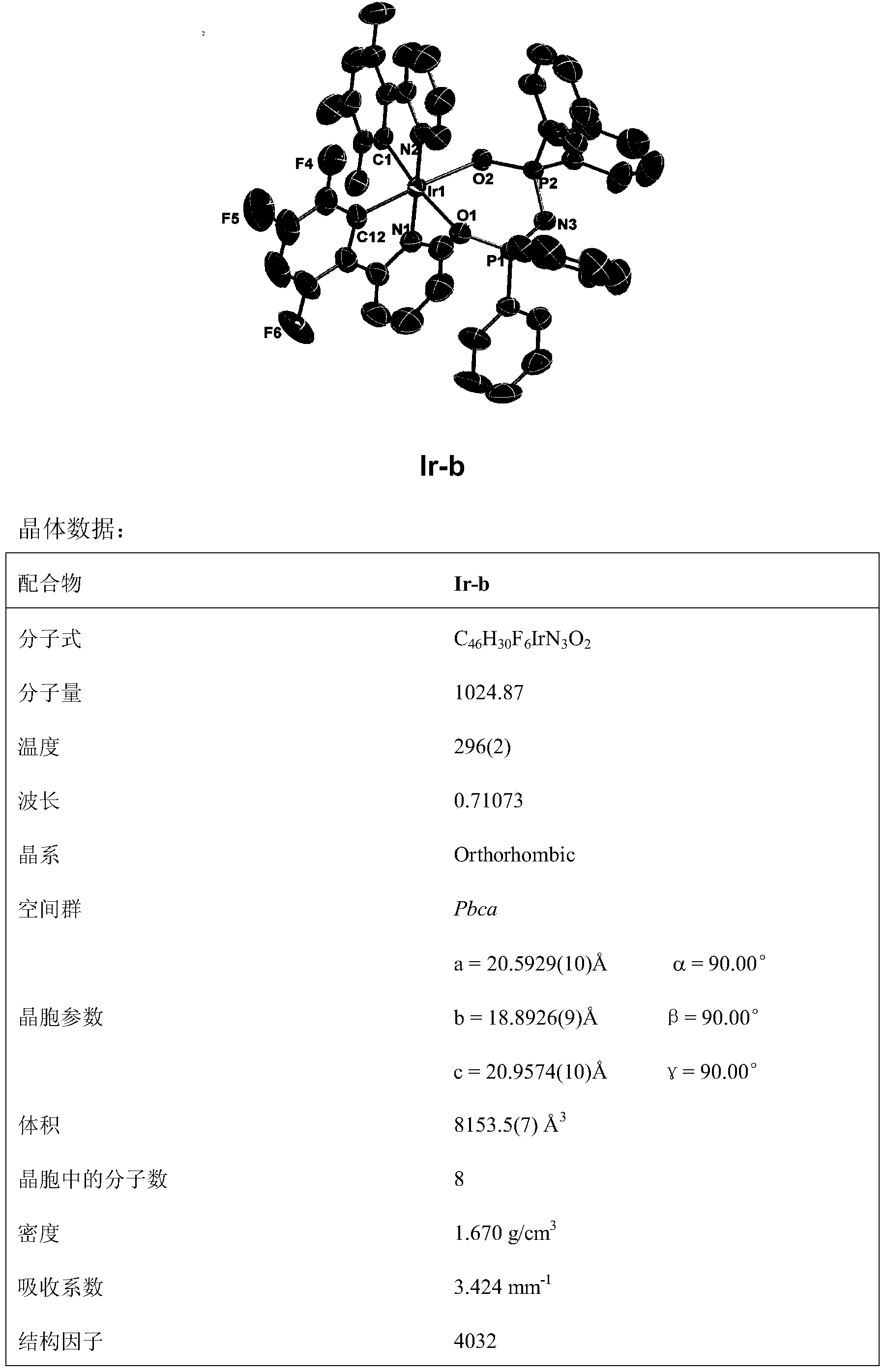
Novel iridium complex and preparation method thereof as well as application thereof in organic electroluminescence device
InactiveCN103450283AGroup 8/9/10/18 element organic compoundsSolid-state devicesOrganic electroluminescenceCoordination complex
The invention discloses an iridium complex with four types of different-site and different-quantity fluoro-substituted 2-phenylpyridine serving as main ligands, and bi(bisubstituent phenyl phosphoro)amine serving as an auxiliary ligand. The formula of the iridium complex is shown in the specification. Emission peaks of an EL (electroluminescence) spectrum of an electroluminescence device prepared from the iridium complex are respectively 497nm, 497nm and 495nm; maximum current efficiencies are respectively 43.95cd / A, 66.36cd / A and 37.36cd / A; maximum power efficiencies are respectively 28.51 lm / W, 48.20 lm / W and 19.11 lm / W; maximum power efficiencies are respectively 38827cd / m<2>, 47627cd / m<2> and 40319cd / m<2>. The invention discloses a preparation method of the iridium complex.
Owner:NANJING UNIV
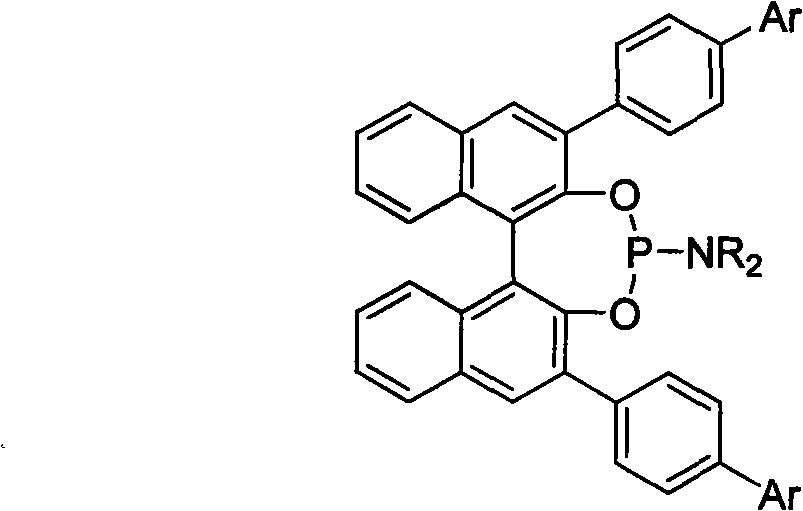
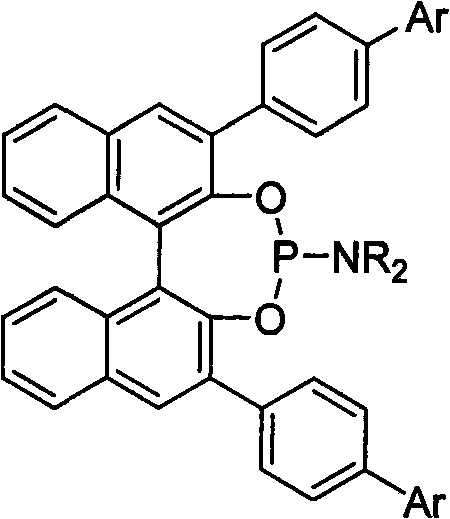
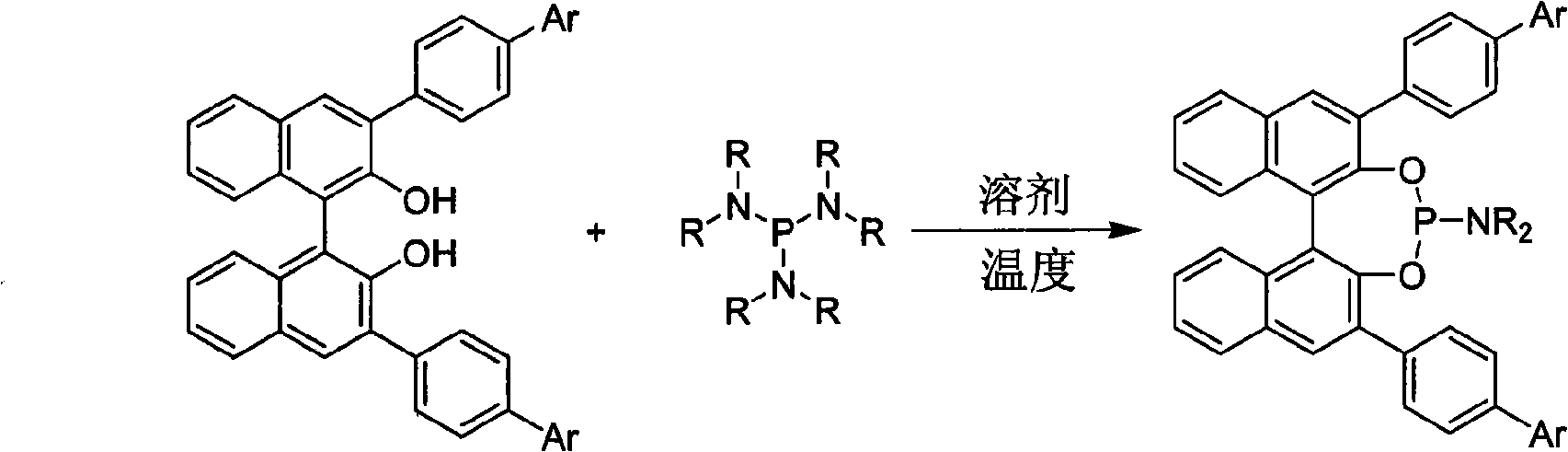
3, 3'-position biaryl group binaphthyl shaft chiral phosphoramidite ligand and preparation method thereof
InactiveCN101565436AGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsOrganic solventPhosphorus trichloride
The invention relates to a 3, 3'-position binaphthyl shaft chiral phosphoramidite ligand containing biaryl group and a preparation method thereof. The preparation method comprises the following steps of: leading 3, 3'-dual-biaryl group-2, 2'-dinaphthol to react with hexagon-alkyl group phosphoramidite in organic solvent at the temperature of 0-120 DEG C according to the mol rate of 1:1, or leading 3, 3'- dual-biaryl group-2, 2'-dinaphthol to react with phosphorus trichloride and dialkyl amine in steps, and after complete reaction, the object chiral phosphoramidite ligand can be obtained through washing, extraction and separation. After going through complexation with metallic copper salt, the chiral phosphoramidite ligand can be used for catalyzing the conjugate addition reaction of zinc alkyl with alpha, beta-unsaturated carbonyl compounds, and an additional product is prepared with high yield up to 78-98% and high enantioselectivity ee value up to 80-98%.
Owner:TIANJIN UNIV
Enantioselective Phosphoramidite Compounds and Catalysts
InactiveUS20070259774A1Easy to getWithout compromising activity and degree of chemical selectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystEnantio selectivity
This invention relates to phosphoramidite compounds and catalyst complexes which can be used to provide enantioselective reactions including hydroamination reactions, etherification reactions and conjugate addition reactions and allylic substitution reactions, among others. In a first aspect, the present invention is directed to phosphoramidite and related compounds according to general structure (I), where Z is absent or is a group containing O, N or S, preferably O; R1 and R2 are independently an optionally substituted C1-12 alkyl group, an optionally substituted (CH2)n-aromatic group or (CH2)n-heteroaromatic group, or are linked together to form an optionally substituted aliphatic or (CH2)n-aromatic dianion of a diol, diamine, dithiol, aminoalcohol, aminohiolate or a alcoholthiol group; R3′ and R3 are each independently H, an optionally substituted C1-C12 alkyl group or an optionally substituted (CH2)n-aromatic group with the proviso that R3′ and R3 are not both H, or together R3′ and R3 form an optionally substituted C5-C15 saturated or unsaturated carbocyclic ring; R4 is H, an optionally substituted C1-C12 alkyl group or an optionally substituted (CH2)n-aromatic group; R5 is absent, H, an optionally substituted C1-C12 alkyl group or an optionally substituted (CH2)n-aromatic or (CH2)n-heteroaromatic group; Ra and Ra′ are each independently H or a C1-C3 alkyl group, or Ra and Ra′ together with the carbon to which they are attached form a optionally substituted C5-C15 saturated or unsaturated carbocyclic or heterocyclic ring, or an aromatic or heteroaromatic ring; R6 and R7 are each independently H, an optionally substituted C1-C12 alkyl group or an optionally substituted (CH2)n-aromatic group, with the proviso that R5, R6 and R7 cannot simultaneously be H, and when Ra and Ra′, together with the carbon to which they are attached, form a carbocyclic ring, heterocyclic ring or an aromatic or heteroaromatic ring, R5 is absent or is preferably H; R6 and R7 are preferably H or CH3; and each n is independently 0, 1, 2, 3, 4, 5 or 6 and wherein at least one of the carbon atoms attached to the nitrogen of the phosphoramidite group is a chiral center.
Owner:YALE UNIV
Phosphoramidites for synthetic RNA in the reverse direction, efficient RNA synthesis and convenient introduction of 3'-end ligands, chromophores and modifications of synthetic RNA
ActiveUS8541569B2Efficient modificationClean oligo synthesisSugar derivativesPeptidesBiologyChromophore
The present invention provides building blocks and methods for synthesizing very pure RNA in a form that can efficiently be modified at the 3′ end. Reverse RNA monomer phosphoramidites have been developed for RNA synthesis in 5′→3′ direction, leading to very clean oligo synthesis that allows for the introduction of various modifications at the 3′-end cleanly and efficiently. Higher coupling efficiency per step have been observed during automated oligo synthesis with the reverse RNA amidites disclosed herein, resulting in a greater ability to achieve higher purity and produce very long oligonucleotides. The use of the reverse RNA phosphoramidites in the synthetic process of this invention leads to oligonucleotides free of N+1 species.
Owner:CHEMGENES CORP
Chromophore-modified deoxynucleoside phosphoramidite monomer compound, preparation method therefor and application thereof
InactiveCN105348343AStability is not affectedDoes not affect the structureSugar derivativesGroup 5/15 element organic compoundsFluorescenceDouble strand
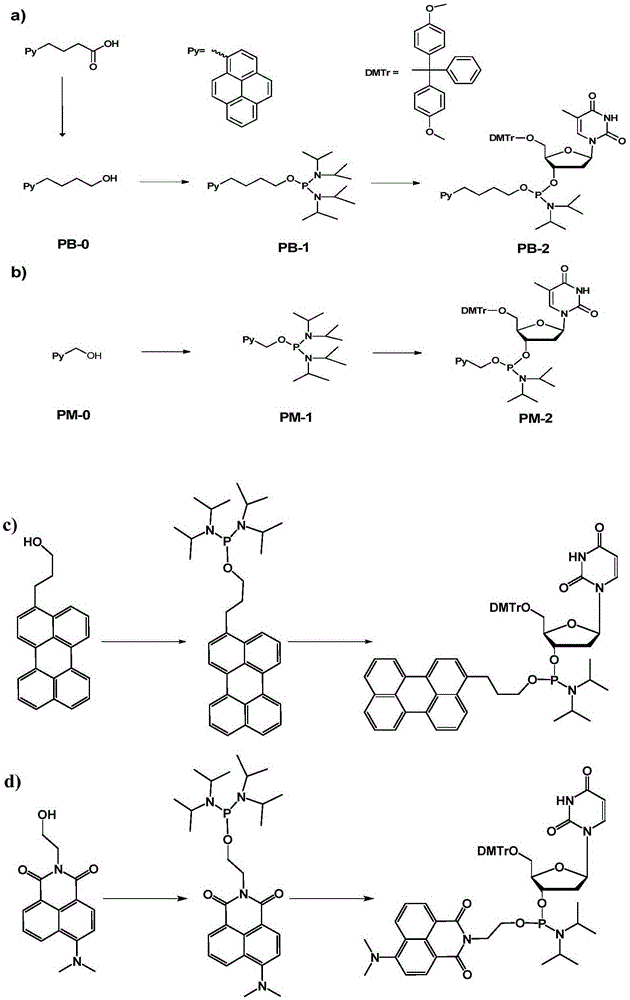
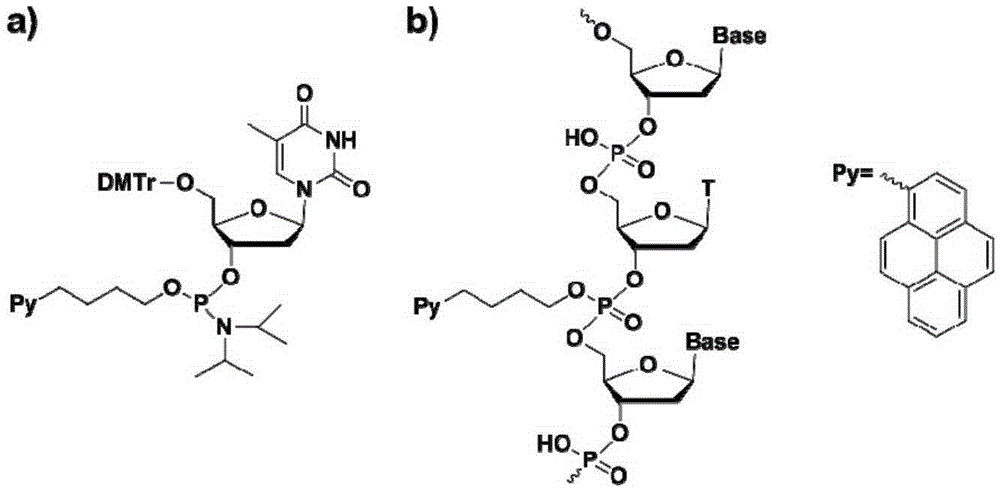
The invention discloses a chromophore-modified deoxynucleoside phosphoramidite monomer compound, a preparation method therefor and an application thereof. The preparation method comprises the steps of: connecting chromophores such as pyrene, perylene or naphthalene carboxamide with bis(diisopropylamino) chlorophosphine to obtain a phosphorous intermediate; and reacting the phosphorous intermediate with DMT-protected deoxynucleoside to obtain a chromophore-modified deoxynucleoside phosphoramidite monomer compound. By virtue of solid-phase synthesis of DNA, the compound is inserted into oligonucleotide at a fixed point to obtain a chromophore-modified fluorescent oligonucleotide probe with a stable double-chain structure. The fluorescent oligonucleotide probe is free of fluorescence-emission, and only being combined with a perfectly matching target chain, the fluorescence can be enhanced by 23.5 times, and the response speed is fast. Mismatched bases are obviously identified with nearly no fluorescence-emission, so that single base mismatch can be obviously identified. The compound can be applied to single base mutation analysis of a gene and detection of a PCR reaction process and the like, and is wide in application prospect in aspects of single base polymorphism detection and nucleic acid detection in a biochemical sample and the like.
Owner:PEKING UNIV
Methyl propenyl phosphoramidite monomer and synthetic method thereof
ActiveCN103819501AHigh purityHigh polymerization efficiencySugar derivativesGroup 5/15 element organic compoundsNitrogen gasSilica gel
The invention relates to a methyl propenyl phosphoramidite monomer and a synthetic method thereof, and relates to a hydrogel. The molecular formula of the methyl propenyl phosphoramidite monomer is C(38+n1)H(50+2n1)N3O6P, wherein n1 is the number of CH2 groups, and n1=1-3. The synthetic method comprises the following steps: firstly preparing acrylic-OH2; then preparing acylic-DMT (Dimethyl Terephthalate); finally mixing the acylic-DMT and N,N-diisopropyl ethyl amine in the presence of nitrogen, then dissolving by using dichloromethane, then adding 2-cyanoethyl N,N-diisopropyl chloro phosphoramidite for reaction, and then carrying out column chromatography isolation by using silica gel to obtain the methyl propenyl phosphoramidite monomer. The methyl propenyl phosphoramidite monomer disclosed by the invention is low-cost in synthetic raw material and simple and feasible in step and can obtain higher polymerization efficiency by being higher in purification efficiency after being synthesized into DNA (Deoxyribonucleic Acid); the methyl propenyl phosphoramidite monomer disclosed by the invention can be embedded into any position of a DNA sequence.
Owner:XIAMEN UNIV
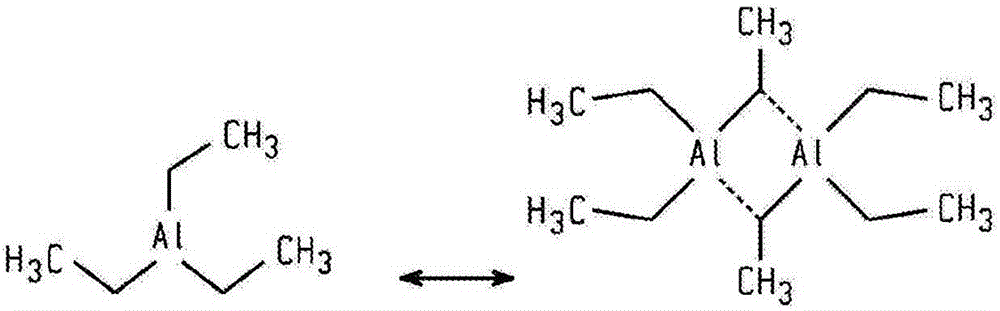
Modifying organoaluminum co-catalysts for improved performance
ActiveCN105980053AOrganic-compounds/hydrides/coordination-complexes catalystsCatalystsCarbamateSilylene
The invention provides modifying organoaluminum co-catalysts for improved performance. Processes of making catalyst compositions are provided. In an exemplary embodiment, the processes include modifying an organoaluminum compound with a modifier that decreases the initial reducing strength of the organoaluminum compound, where the modifier can be an ether, an anhydride, an amine, an amide, a silicate, a silyl ether, a siloxane, an ester, a carbonate, a urea, a carbamate, a sulfoxide, a sulfone, a phosphoramide, or a combination thereof. The processes further include adding a transition metal complex to the mixture of the organoaluminum compound and the modifier; and obtaining a catalyst composition including the organoaluminum compound and the transition metal complex.
Owner:SABIC GLOBAL TECH BV
Reactive phosphorus-containing flame retardant and preparation method and application thereof
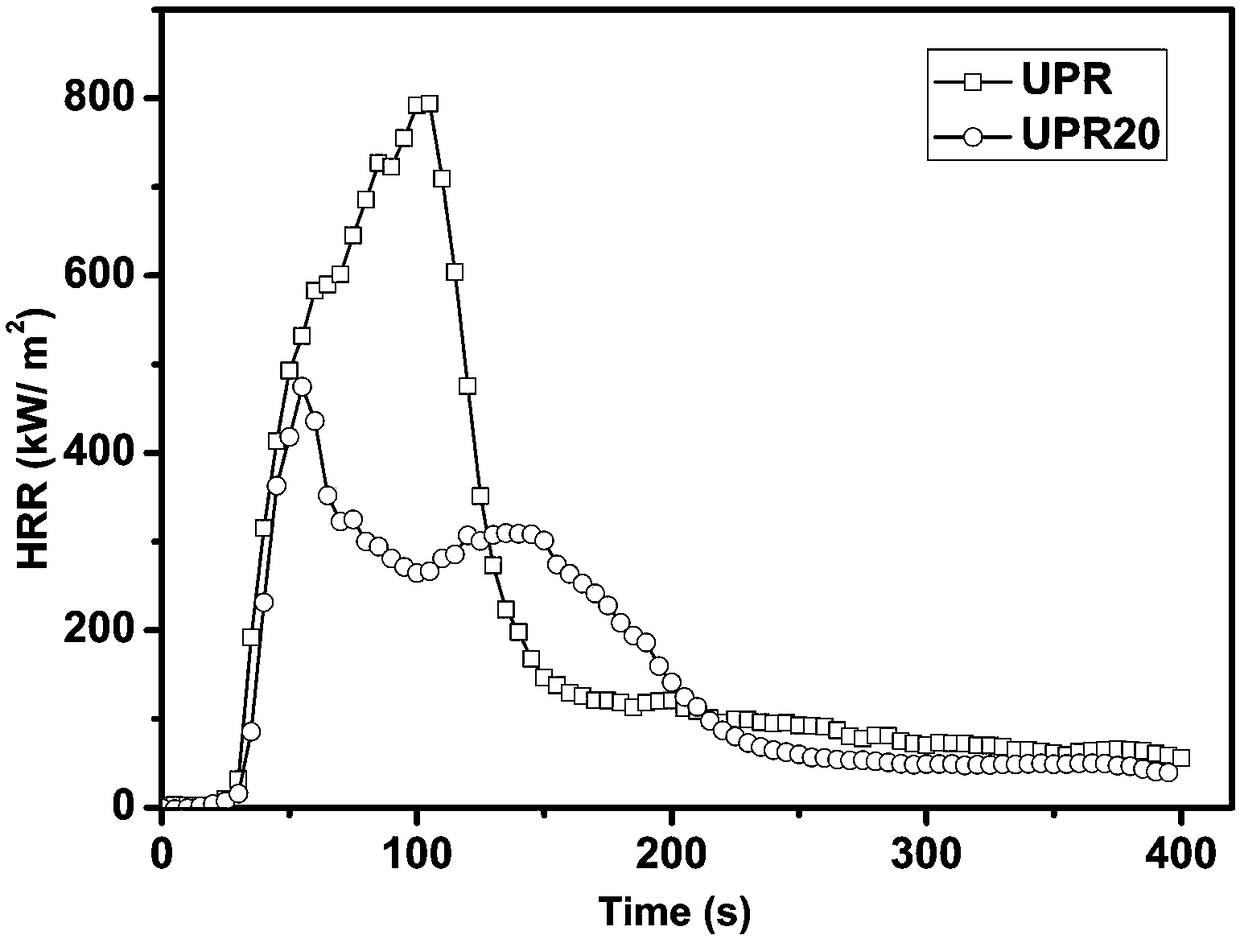
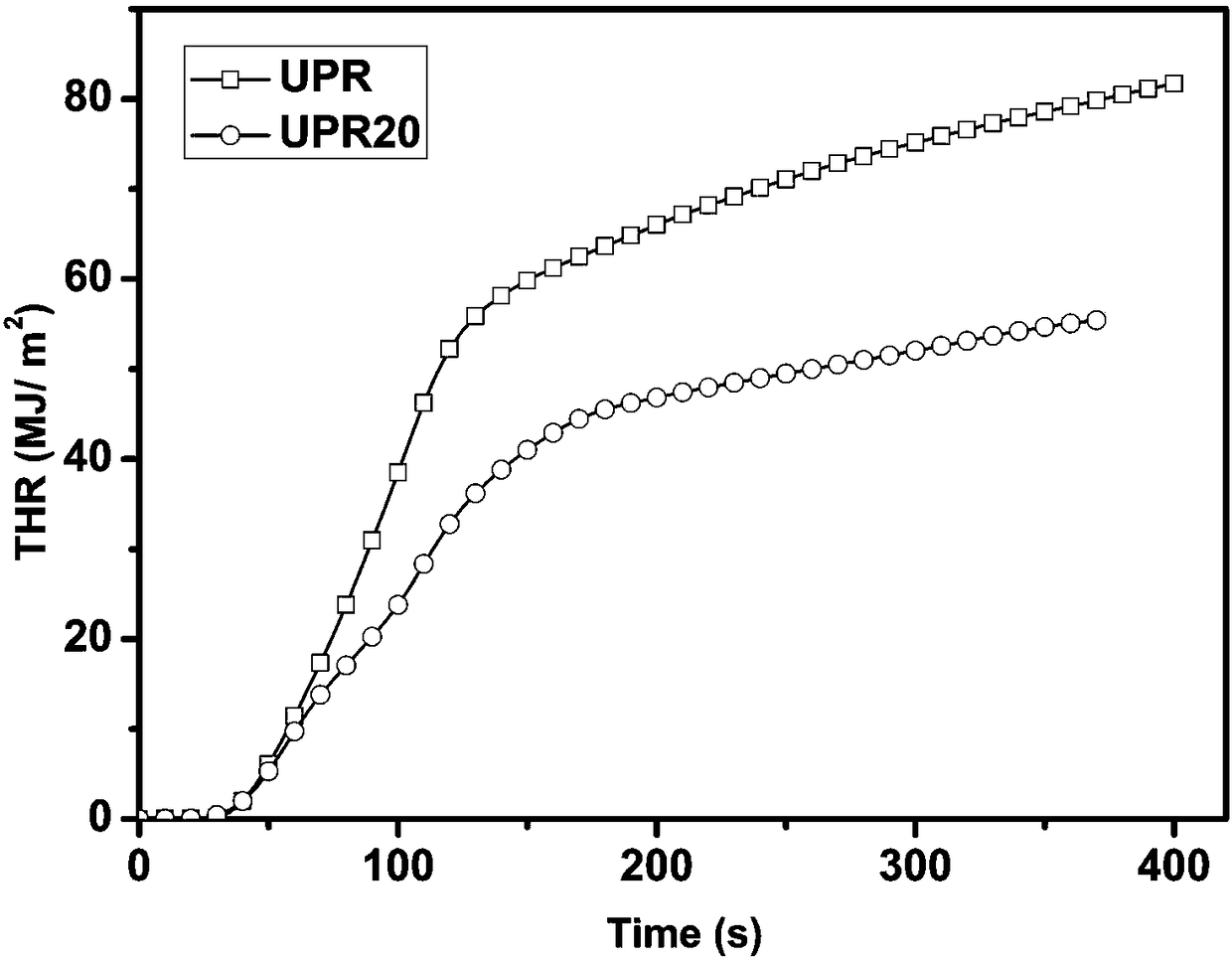
The invention provides a reactive phosphorus-containing flame retardant. The flame retardant contains unsaturated double bonds and can react with polymer, and phosphate and phosphamide structural units are further introduced based on the structure with the unsaturated double bonds. After the obtained flame retardant is applied to unsaturated polyester resin, the highest level of vertical burning UL-94 reaches the level of V-1-V-0, the maximum value of the limit oxygen index LOI is 33.0%, and the peak of heat release rate (PHRR) and total heat release (THR) in cone calorimetry tests are lower than the PHRR and THR of pure unsaturated polyester; after the obtained flame retardant is applied to unsaturated polyester glass fiber composite materials, the highest level of vertical burning UL-94reaches the level of V-1-V-0, and the maximum value of the limit oxygen index LOI is 40%. The reactive phosphorus-containing flame retardant has good flame retardancy and little influences on the mechanical properties of the materials. The invention also provides a preparation method of the flame retardant. The preparation method of the flame retardant is simple, and has mild conditions and easy control.
Owner:SICHUAN UNIV
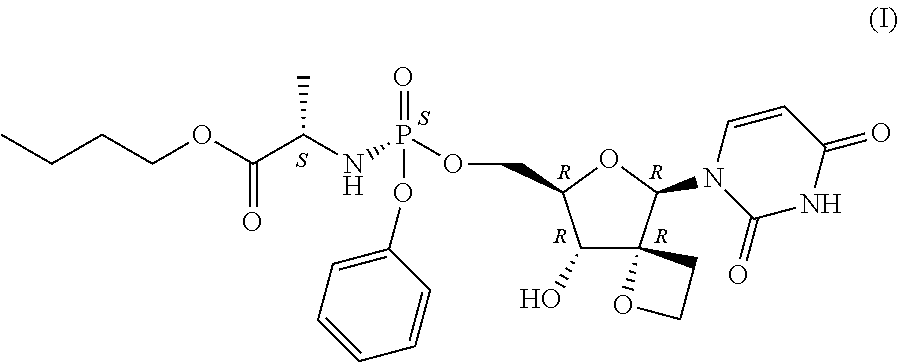
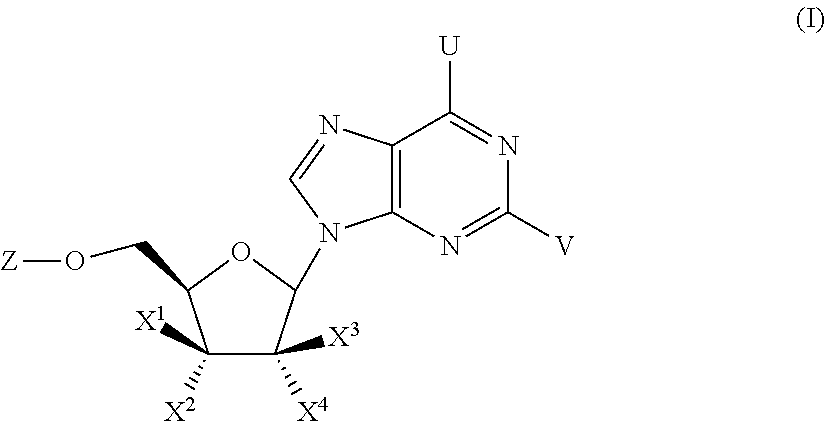
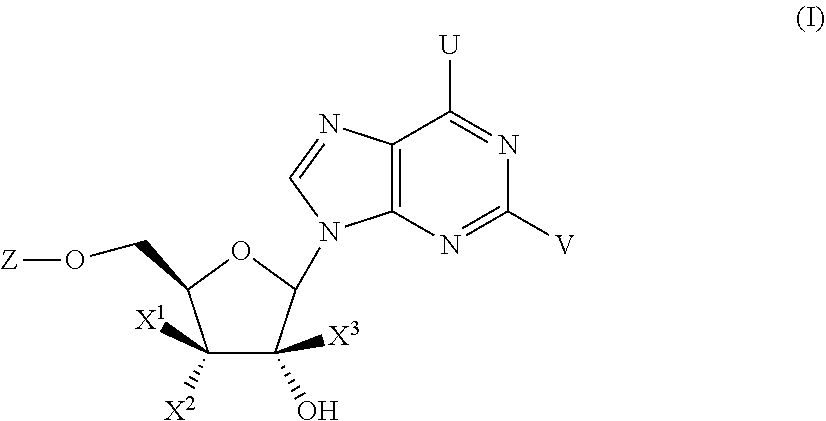
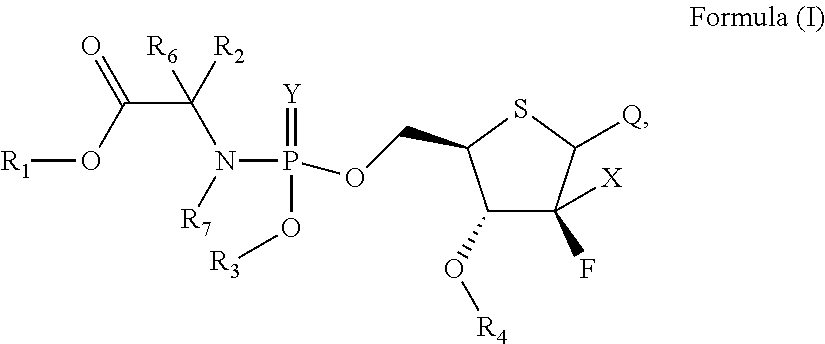
Substituted Purine Nucleosides, Phosphoramidate and Phosphordiamidate Derivatives for Treatment if Viral Infections
This invention is directed to compounds of Formula (I) having the structure that are useful in the treatment of viral infections in mammals, particularly in humans, mediated, at least in part, by a virus in the Flaviviridae family of viruses.
Owner:UNIV COLLEGE CARDIFF CONSULTANTS LTD +1
Oligonucleotide manufacturing method
InactiveUS20180291056A1Efficient and stable productionEfficient productionSugar derivativesBulk chemical productionProtecting groupPhosphoramides
The present invention aims to provide a more stable and efficient method for producing oligonucleotide, particularly, oligonucleotide having various functional groups linked to the 3′-terminal and the like. Efficient production of oligonucleotide becomes possible by a production method of an oligonucleotide represented by the formula (Ia-2) (each symbol is as defined in the DESCRIPTION) and having a functional group at the 3′-terminal, the method including a step of subjecting an oligonucleic acid with 3′-terminal protected by a silyl-protecting group to 3′-terminal-selective deprotection under desilylation conditions that do not affect protecting groups other than the silyl group, subjecting same to phosphitylation conditions with a phosphoramidite reagent that do not affect protecting groups on the oligonucleic acid to give a 3′-terminal-phosphoramidited oligonucleotide represented by the formula (Ia-1) (each symbol is as defined in the DESCRIPTION), and linking a functional group to the 3′-terminal of the 3′-terminal phosphoramidited oligonucleotide directly or via a linker, and the like.
Owner:AJINOMOTO CO INC
Method and antisense composition for selective inhibition of HIV infection in hematopoietic cells
The invention provides antisense antiviral compounds and methods of their use in inhibition of growth of human immunodeficiency virus-1 (HOV-1), as in treatment of a viral infection. The antisense antiviral compounds have morpholino subunits linked by uncharged phosphorodiamidate linkages interspersed with cationic phosphorodiamidate linkages. An exemplary embodiment of the invention provides an antisense compound directed to the HIV Vif gene, causing the production of defective HIV- 1 virions in an infected individual.
Owner:AVI BIOPHARMA
Aryl-substituted phosphonaminate and application in medical science thereof
The invention relates to an aryl-substituted phosphonaminate and an application of the aryl-substituted phosphonaminate in medical science, in particular to an aryl-substituted phosphonaminate showed in a general formula (I), stereoisomer of the aryl-substituted phosphonaminate or acceptable salt in the medical science, a preparation method of the aryl-substituted phosphonaminate, a pharmaceutical composite containing with the aryl-substituted phosphonaminate, the stereoisomer and the acceptable salt and an application for preparing medicine curing virus-infectious diseases of the pharmaceutical composite.
Owner:SICHUAN HAISCO PHARMA CO LTD
Sulfur octyl phosphoramido ester and synthetic method and application thereof
InactiveCN107674094AImprove universalityImprove stabilityGroup 5/15 element organic compoundsDNA preparationChemical synthesisEnd-group
The invention relates to the technical field of chemical synthesis, in particular to thio-octyl phosphoramido ester and a synthetic method and application thereof. The synthetic method of the thio-octyl phosphoramido ester includes the step of: adopting thioctic acid as a raw material, performing a reduction reaction to obtain thio-octanol, and performing an esterification reaction to obtain the thio-octyl phosphoramido ester. When the thio-octyl phosphoramido ester is used as a DNA end-group modifier, DNA with sulfur-containing modified end groups can be prepared efficiently and massively, and the simple and efficient DNA end-group modifier with controllable cost can be provided for sulfur-containing modification of a 5' end of DNA; meanwhile, the synthetic method has the advantages of aconcise path, convenient operation, a simple method for purifying intermediates and the product, and requirements of scientific research and business can be fully met.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
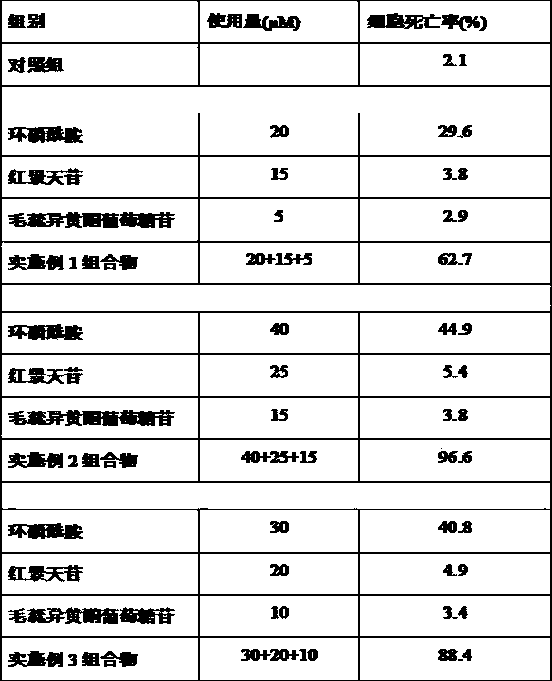
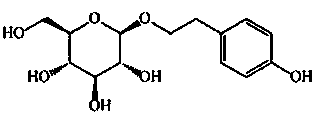
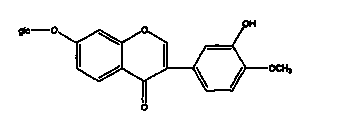
Cyclophosphamide-containing pharmaceutical composition and application in treatment for breast cancer
The invention belongs to the field of medicines, and particularly relates to a cyclophosphamide-containing pharmaceutical composition, and an application thereof in treatment for cancer, in particular for breast cancer. The pharmaceutical composition is composed of cyclophosphamide, salidroside and calycosin-7-oxy-beya-D-glucopyranoside, wherein the molar ratio of cyclophosphamide to salidroside to calycosin-7-oxy-beya-D-glucopyranoside is (20-40): (15-25): (5-15). The pharmaceutical composition disclosed by the invention is capable of reducing the toxic and side effects of cyclophosphamide and remarkably enhancing the curative effect, provides a new thought and strategy for treatment of breast cancer, and has important clinical significance and wide application prospect.
Owner:徐鹏
Non-aqueous electrolyte for lithium ion battery and lithium ion battery thereof
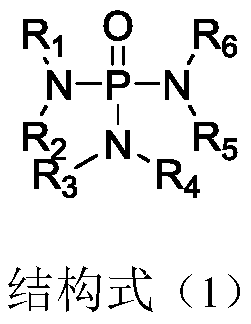
PendingCN110797574ALess side effectsImprove stabilitySecondary cells servicing/maintenanceOrganic electrolytesElectrolytic agentAlkane
The invention discloses a non-aqueous electrolyte for a lithium ion battery and the lithium ion battery. The electrolyte comprises electrolyte lithium salt lithium hexafluorophosphate, a non-aqueous organic solvent and an additive; the additive comprises an additive A which is a phosphamide compound with a structural formula (1); the structural formula (1) is shown in the specification, in the structural formula (1), each of R1 to R6 is respectively and independently one of alkane groups or halogenated alkane groups with the carbon atom number of 1 to 5; and the additive amount is 0.1%-10% ofthe total weight of the electrolyte. The additive A provided by the invention can form a stable complexing state with hexafluorophosphate ions in a lithium hexafluorophosphate electrolyte system, so that the hexafluorophosphate anions exist more stably, battery side reactions caused by decomposition of the hexafluorophosphate anions are reduced, and the stability of the electrolyte is improved; and the electrolyte can be combined with other common additives to play a synergistic role, so that the lithium ion battery adopting the electrolyte has better electrical cycle performance.
Owner:桑顿新能源科技(长沙)有限公司
Novel compound of 4'-thionucleoside, as well as preparation method therefor, pharmaceutical composition thereof and application thereof
ActiveUS20180079770A1Organic active ingredientsSugar derivativesAbnormal tissue growthThionucleosides
The present invention relates to a novel compound of 4′-thionucleoside, a preparation method therefor, a pharmaceutical composition comprising the same and an application thereof. Specifically, the present invention relates to a phosphamide derivative of 4′-thionucleoside, a preparation method therefor, a pharmaceutical composition comprising the same, a use thereof in the preparation of a medicine for preventing or treating abnormal cell proliferation diseases (for example, tumors or cancers and related diseases) or virus infectious diseases, and a method of using the same for preventing or treating abnormal cell proliferation diseases (for example, tumors or cancers and related diseases) or virus infectious diseases.
Owner:SICHUAN KELUN BIOTECH BIOPHARMACEUTICAL CO LTD
Matrix for cultivating Sparassis crispa
ActiveCN106365800AReduce churnHigh yieldCalcareous fertilisersAlkali orthophosphate fertiliserGlucono delta-lactonePhosphate
The invention discloses a matrix for cultivating Sparassis crispa. The matrix is prepared from the following components (in parts by weight) including a vector, a nutrient and water; the vector is prepared from the following components (in parts by weight) including an acrylic monomer, oxamide, p-ethoxy benzamide, 4-hydroperoxyl cyclophosphamide, tris (butoxyethyl) phosphate, a crosslinking agent, an initiator, a reinforcing agent, maleamic acid and phosphoenolpyruvate; and the nutrient is prepared from the following components (in parts by weight) including starch, dextran, vitamin B1, ammonium phosphate, peptone, D-glucose-6-phosphate, glucono-delta-lactone, glucosamine sulfate sodium, EDTA calcium disodium, sodium propionate and sodium dihydroxyethylglycinate. The prepared matrix can have better preservation action on the nutrient, thus enabling the Sparassis crispa to better grow and have higher yield.
Owner:福建容益菌业科技研发有限公司
Mesoporous molecular sieve/phosphoramide composite material and preparation method thereof
InactiveCN105478077ALarge specific surface areaHigh affinityOther chemical processesMolecular sieveCovalent Interaction
The invention discloses a mesoporous molecular sieve / phosphoramide composite material and a preparation method thereof. The mesoporous molecular sieve / phosphoramide composite material aims to solve the problems that existing SBA-15 molecular sieves are poor in uranyl adsorbing capacity. The mesoporous molecular sieve / phosphoramide composite material comprises components with percentage by mass: 75-97% of SBA-15 mesoporous molecular sieves and 3-25% of phosphamide perssad, wherein the phosphamide perssad and the SBA-15 are connected through covalent interaction. The invention substantially discloses an SBA-15 mesoporous molecular sieve- phosphoramide composite material and a preparation method thereof, the mesoporous molecular sieve / phosphoramide composite material has large specific surface area and excellent appetency to the uranyl, has excellent uranyl ion adsorption property, and can effectively solve the problem that existing SBA-15 molecular sieves are poor in uranyl adsorbing capacity. Simultaneously, the preparation method of the mesoporous molecular sieve / phosphoramide composite material is simple, low in production cost and high in yield, can meet the demands of large-scale industrialized application, has excellent application prospect, and is worthy of large-scale popularization and application.
Owner:MATERIAL INST OF CHINA ACADEMY OF ENG PHYSICS
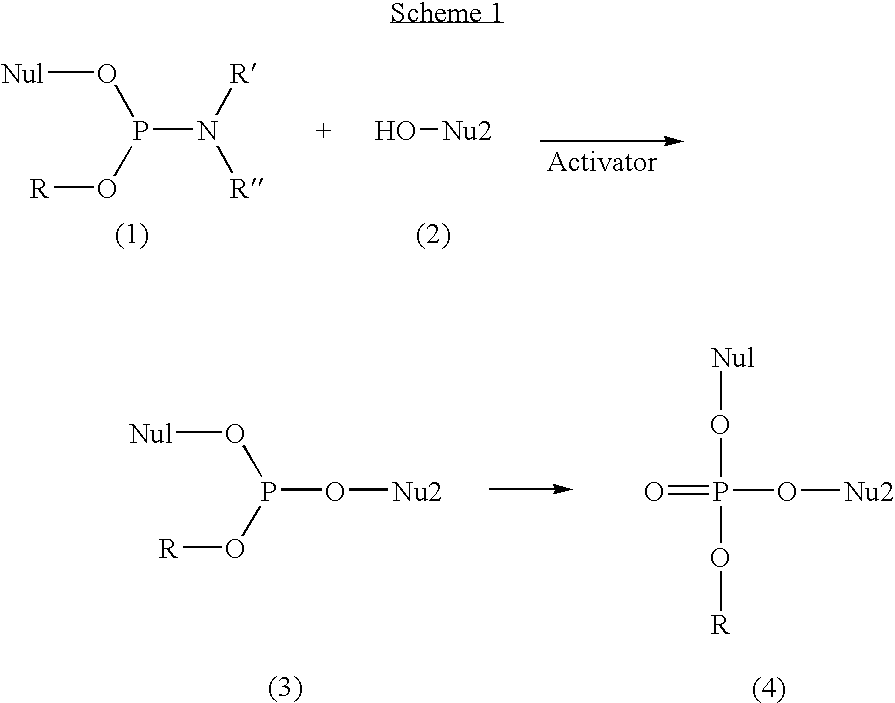
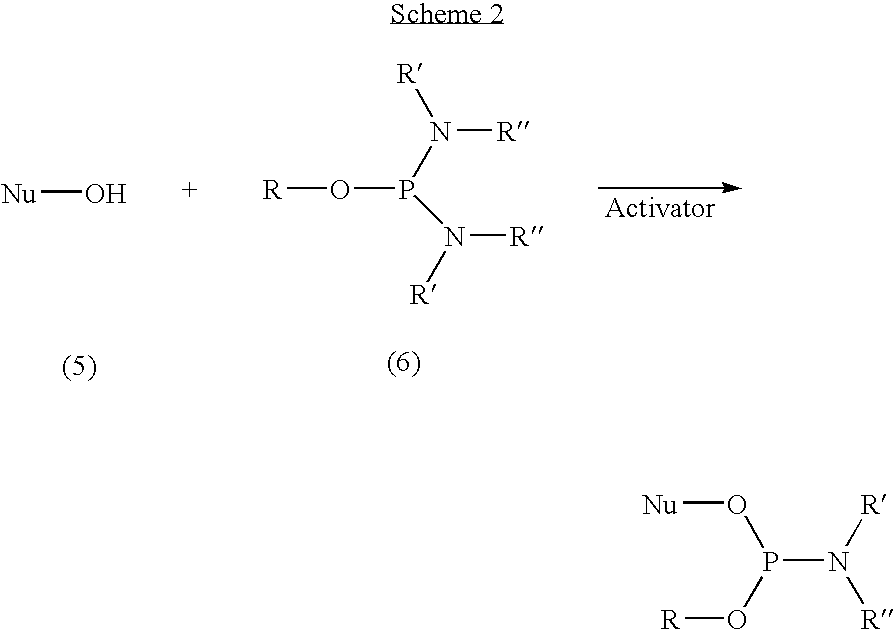
Activators for oligonucleotide and phosphoramidite synthesis
The present invention discloses novel methods for the synthesis of oligonucleotides and nucleoside phosphoramidites. The methods are based on employing aryl-substituted 5-phenyl-1H-tetrazoles with perfluoroalkyl groups on the aromatic ring as activators. In one aspect the novel activators are used in the synthesis of oligonucleotides via the phosphoramidite approach. In this aspect the activators are highly efficient and can be applied with very short coupling times. In a further aspect, the activators of the invention are used in the synthesis of phosphoramidites through the reaction of nucleosides comprising a free hydroxyl group with phosphitylating agents. In this aspect the activators provide very pure phosphoramidites under mild conditions. The activators of the invention are further characterized by being highly soluble, non-hygroscopic and non-hazardous.
Owner:SIGMA ALDRICH CO LLC
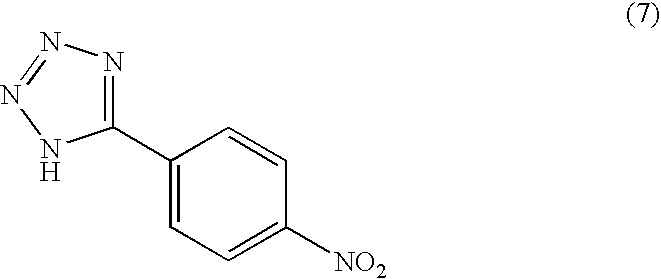
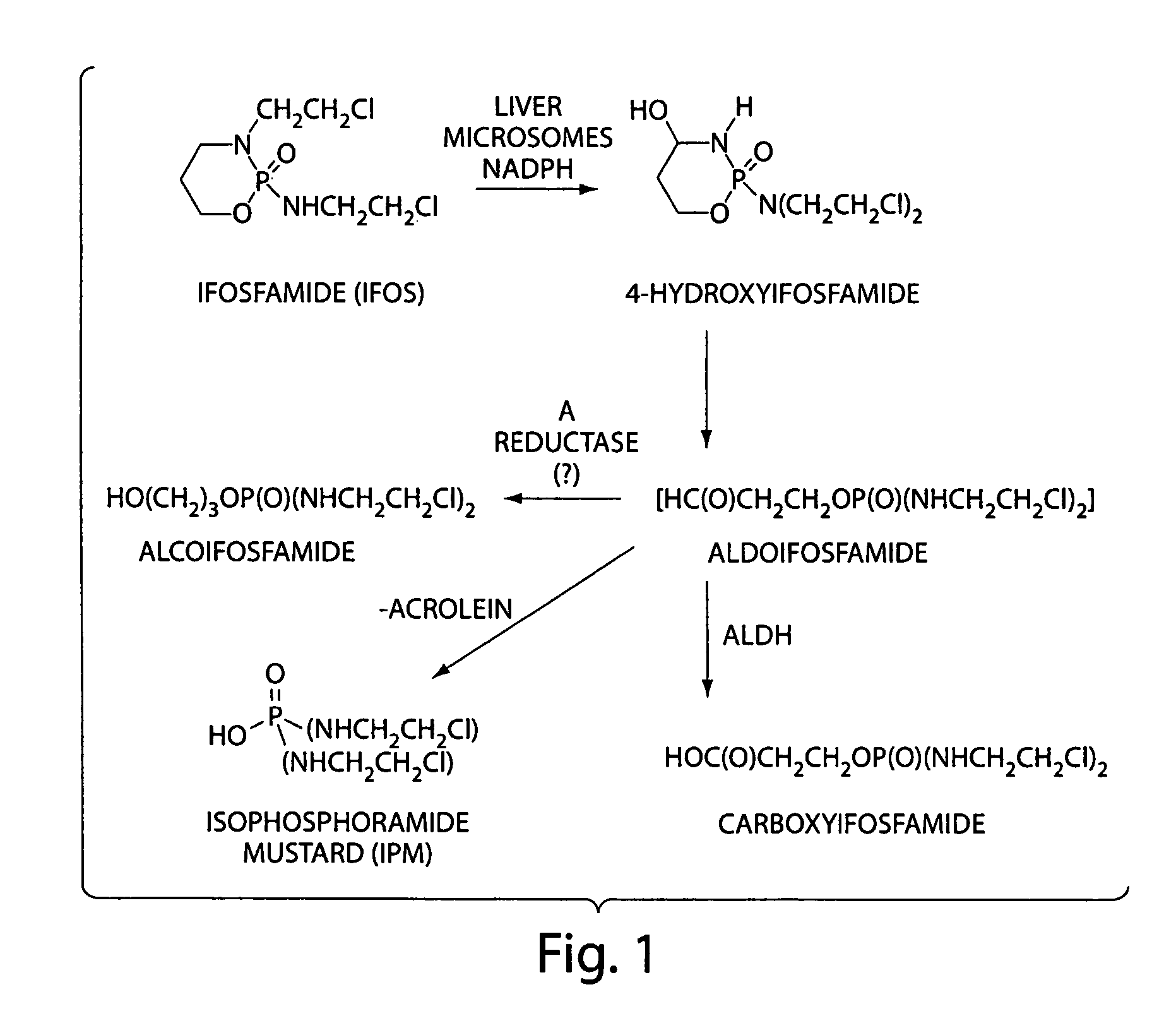
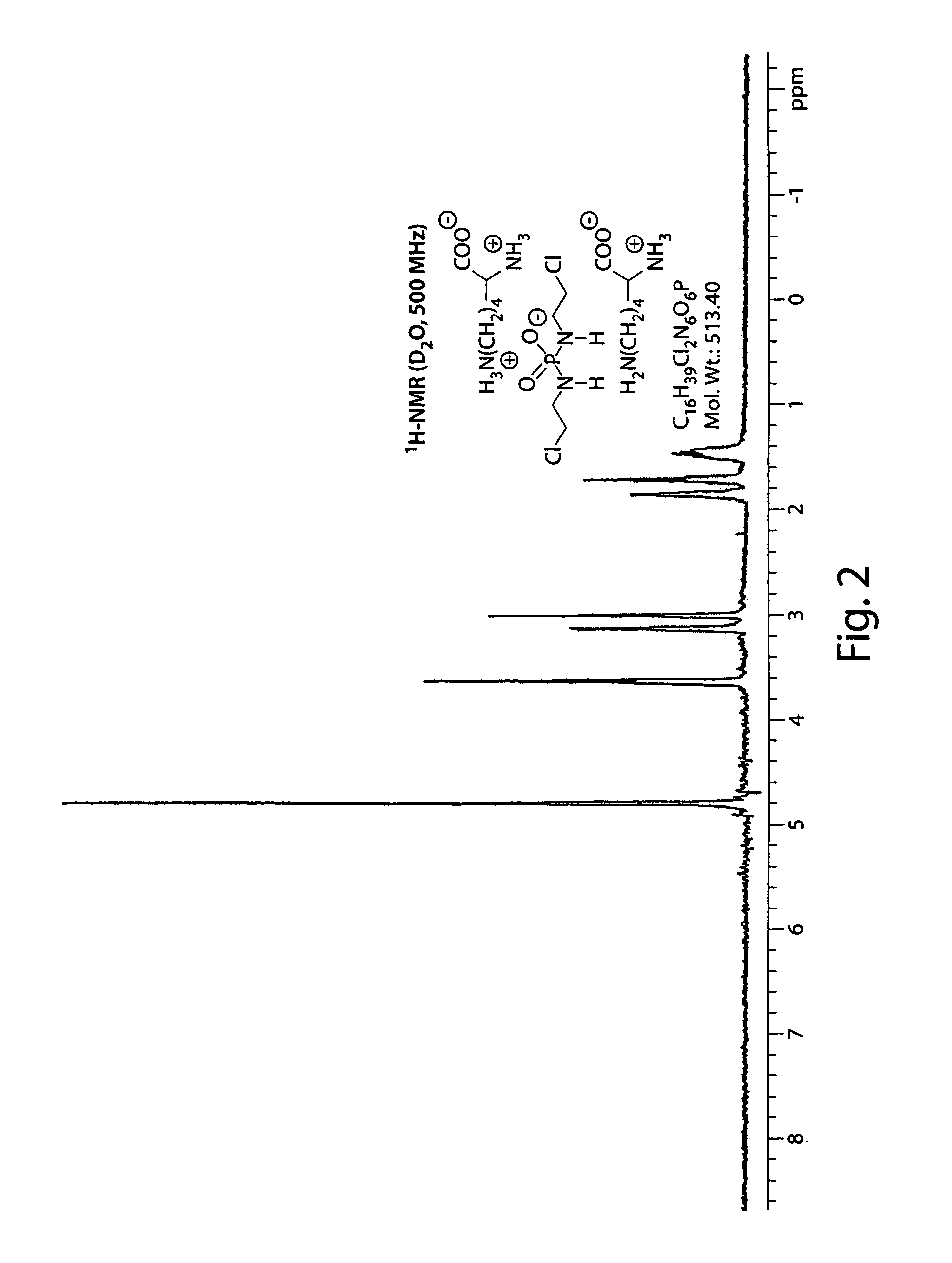
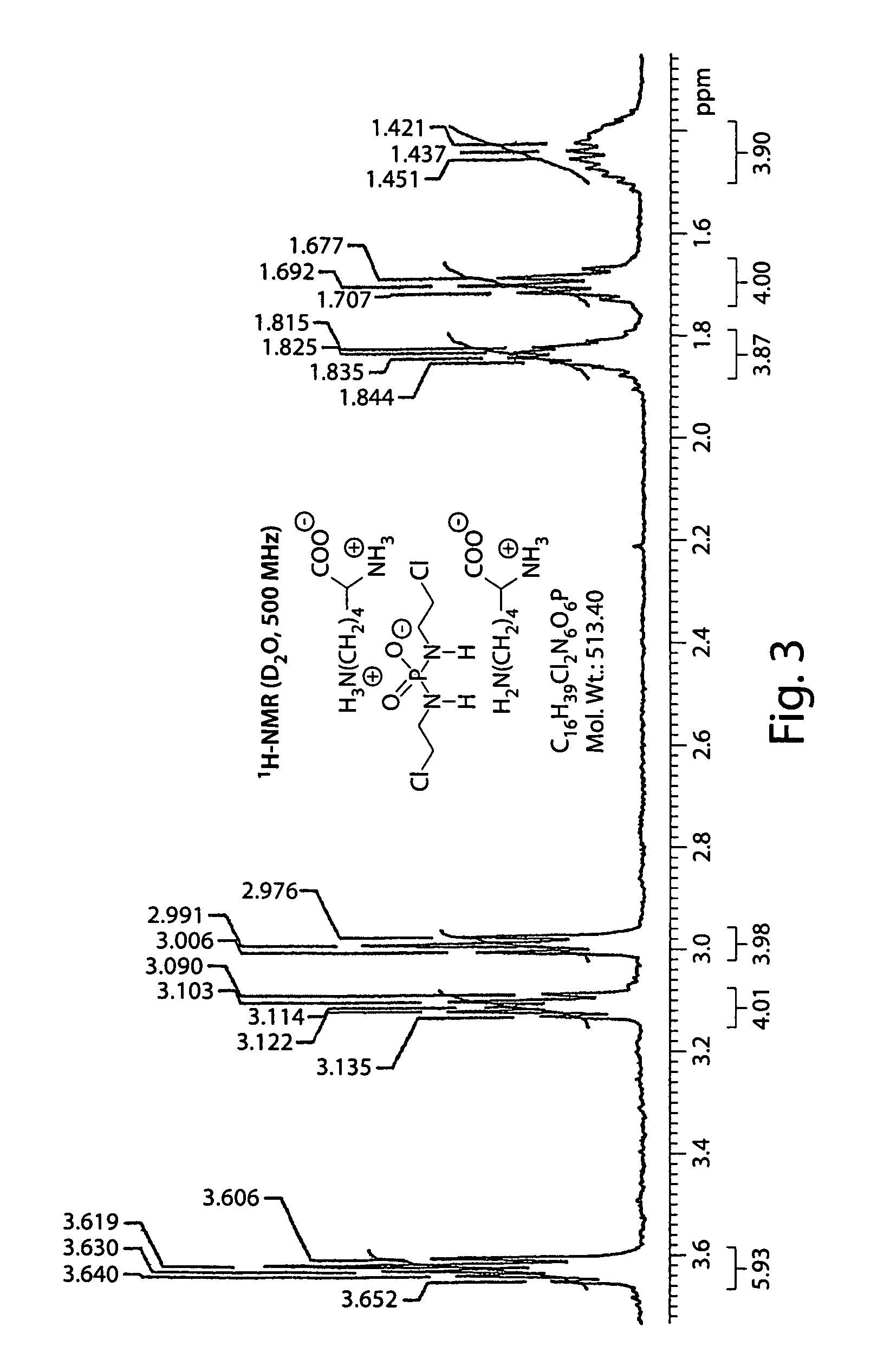
Salts of isophosphoramide mustard and analogs thereof as anti-tumor agents
The present disclosure relates to salts and compositions of isophosphoramide mustard and isophosphoramide mustard analogs. In one embodiment the salts can be represented by the formulawherein A+ represents an ammonium species selected from the protonated (conjugate acid) or quaternary forms of aliphatic amines and aromatic amines, including basic amino acids, heterocyclic amines, substituted and unsubstituted pyridines, guanidines and amidines; and X and Y independently represent leaving groups. Also disclosed herein are methods for making such compounds and formulating pharmaceutical compositions thereof. Methods for administering the disclosed compounds to subjects, particularly to treat hyperproliferative disorders, also are disclosed.
Owner:DEKK TEC INC
Process for manufacturing purified phosphorodiamidite
InactiveUS7057062B2Improved yield and purityReduce impuritySugar derivativesChemical recyclingOrganic solventImpurity
A scalable process for purification of phosphorodiamidite includes steps of solubilizing a crude phosphorodiamidite in an apolar organic solvent, contacting the non-polar organic solvent with a polar phase comprising a polar organic solvent to remove impurities from the solubilized phosphorodiamidite, and removing the non-polar organic solvent from the phosphorodiamidite.
Owner:IONIS PHARMA INC
Method for synthesizing phosphoramidon by utilizing hydrogen phosphorous acid diester intermediate
InactiveCN103242432ASimplify deprotection stepsHigh yieldPeptide preparation methodsBulk chemical productionPhosphorous acidChemical synthesis
The invention relates to a method for synthesizing phosphoramidon by utilizing a hydrogen phosphorous acid diester intermediate. The method comprises the following steps of: generating a phosphoramidite intermediate under the alkaline condition by utilizing alpha-L-triacetyl rhamnose and benzyloxy-diisopropyl amino phosphorus oxychloride; carrying out catalytic hydrolysis with weak acid to obtain alpha-L-rhamnose-1-hydrogen phosphorous acid diester intermediate; reacting with a nitrogen terminal of leucine-tryptophan dipeptide benzyl ester by utilizing an oxidation coupling method to obtain a phosphamide precursor; and finally carrying out 'one-pot' hydrogenation and deacetylation to remove all the protecting groups of the phosphamide precursor, and carrying out chromatographic purification by virtue of glucose gel to obtain high-purity phosphoramidon. According to the method, the protecting groups of three synthesis fragments, namely L-rhamnose, phosphorylation reagent and leucine-tryptophan dipeptide, are designed and optimized, so that a step of removing protection of a product precursor is simplified to the utmost extent; and yield of a target product is greatly increased by utilizing a novel hydrogen phosphorous acid diester intermediate and the oxidation coupling method, so that a new method is established for chemical synthesis of phosphoramidon.
Owner:JIANGXI SCI & TECH NORMAL UNIV
N-acylphosphoramidites and their use in oligonucleotide synthesis
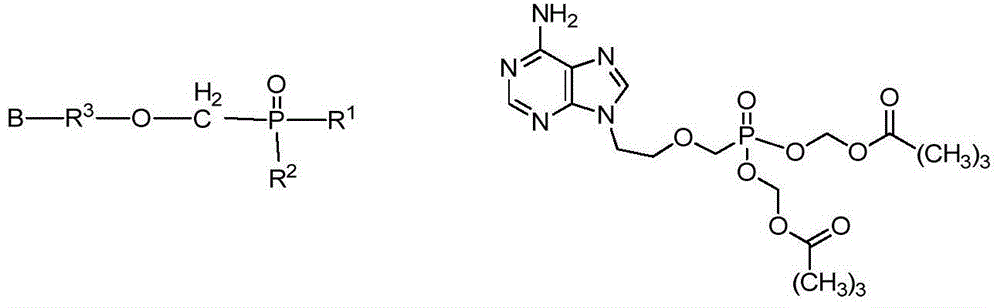
The present invention provides a compound of formula (I), (II), or (III), wherein R1, R2, R2′, R3, and R3′ are the same or different and each is H an alkyl, an alkenyl, an alkynyl, a cycloalkyl, an aryl, or an aralkyl. Alternatively, either of R2 or R2′ combined with either of R3 or R3′ comprises a ring. R4 is a protecting group or a solid support R5 is H or an alkyl. R6 is a protecting group, an amidoalkyl, an alkyl, an alkyl ketone, an alkenyl, an alkynyl, a cycloalkyl, an aryl, or an aralkyl. R15 is H or a protecting group. Q and Q1 are the same or different and each is a nucleoside, an oligonucleotide comprising a nucleoside, or an oligomer comprising a nucleoside, which is of formula (a) or (b), wherein B is a labeling group, an alkyl, an alkenyl, an alkynyl, a cyclic group optionally containing one or more heteroatoms, or an amino; and, E is H, a halogen, a hydroxy, an alkoxy, an ester, an amino or a protecting group. X and X1 are independently O, S, or Se, and n is an integer from 1 to about 300. Each Q in each monomeric unit defined by n can be the same or different. The present invention further provides a method of preparing a polymer using the N-acylphosphoramidite of formula (I) or (II).
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC
Phosphoramidite monomer composition and preparation method and application thereof
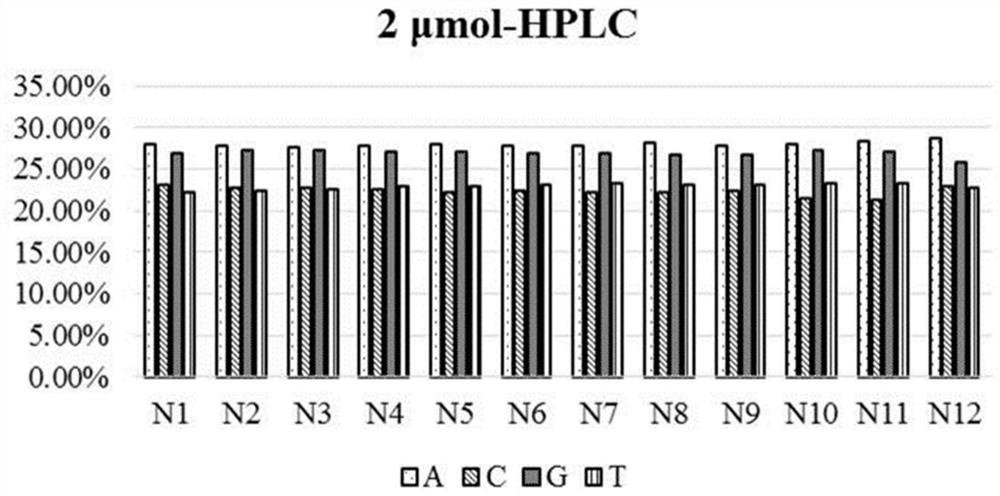
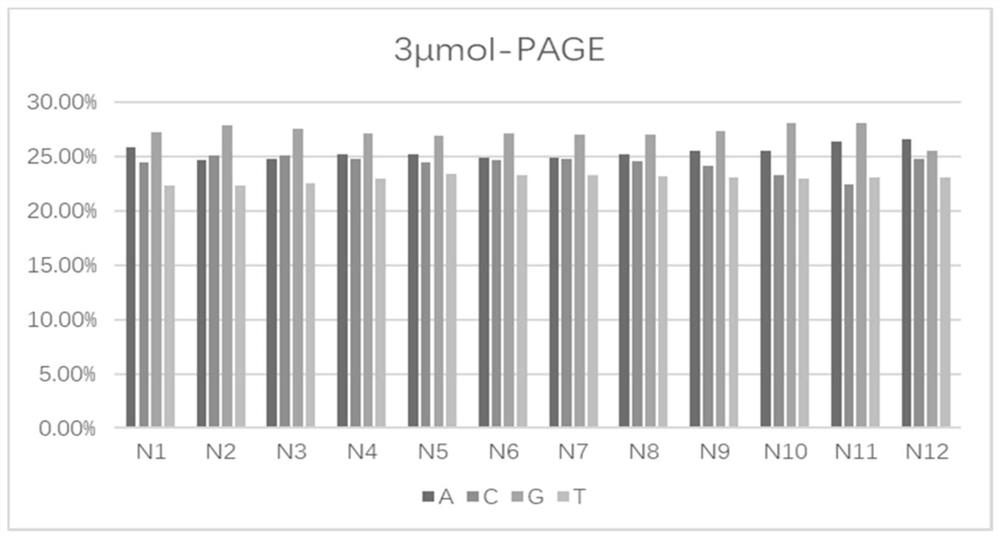
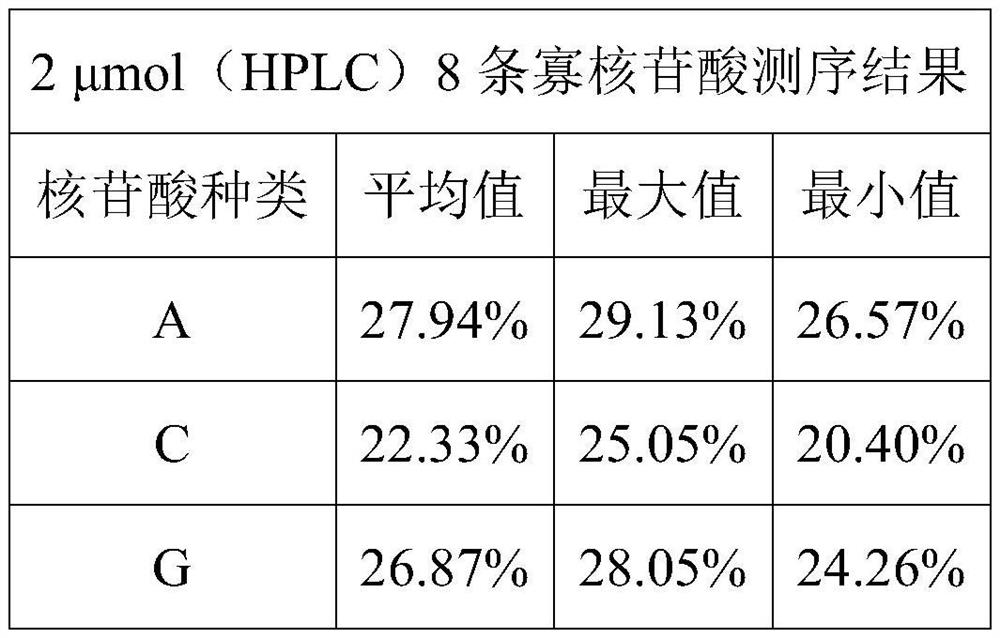
PendingCN112898363AAccurate distributionPrecise control ratioSugar derivativesSugar derivatives preparationMonomer compositionPhysical chemistry
The invention provides a phosphoramidite monomer composition and a preparation method and application thereof. The phosphoramidite monomer composition comprises a dA solution, a dC solution, a dG solution and a dT solution, wherein the concentration of the dA solution is 0.01-0.05g / mL; the concentration of the dC solution is 0.01-0.03g / mL; the concentration of the dG solution is 0.01-0.03g / mL; the concentration of the dT solution is 0.01-0.03g / mL; and the volume ratio of the dA solution to the dC solution to the dG solution to the dT solution is (5-6) to (4-5.1) to (4-5.1) to (4-5). The phosphoramidite monomer composition provided by the invention is reasonable in proportion; the distribution of degenerate sites in prepared oligonucleotide is reasonable; and the degeneracy of each degenerate site is within the range of 20-30%.
Owner:GENEWIZ INC SZ
Method for preparing brown ginseng extract with high antitumor activity and application of method
InactiveCN104523789AImprove anti-tumor activityFast preparationAntineoplastic agentsPlant ingredientsSide effectGINSENG EXTRACT
The invention discloses a method for preparing a brown ginseng extract with high antitumor activity and an application of the method. The whole process is characterized in that the brown ginseng extract with high antitumor activity is quickly and conveniently prepared finally by the steps of enzymatic hydrolysis, high-temperature high-pressure heating, crushing, extraction and the like. Compared with untreated ginseng extracts, the activity of the extract obtained is increased by about 80 times. Cytotoxicity test and animal model experiment results show that compared with untreated common ginsengs, the brown ginseng extract has relatively high anti-tumor and combined anti-tumor activity, has an obvious inhibiting effect on formation of cyclophosphamide-induced bone marrow micronucleus of tumor-bearing mice and has a remarkable improving effect on immunosuppressive action induced by cyclophosphamide, which shows that the brown ginseng extract combined with cyclophosphamide in use can inhibit the toxic and side effects of cyclophosphamide and promote clinical application of cyclophosphamide.
Owner:吉林玉参医药科技有限公司
Method for reducing fluorescent background of Taqman probe
PendingCN112979737APrevent participation in follow-up responsesAvoid problemsSugar derivativesMicrobiological testing/measurementCombinatorial chemistryOrganic chemistry
The invention discloses a method for reducing the fluorescent background of a Taqman probe. The method comprises the following steps: when a solid-phase phosphoramidite triester method is adopted to synthesize the Taqman probe on a full-automatic DNA synthesizer, firstly capping a 3-terminal quenching group CPG, then carrying out a four-step circular reaction of deprotection, coupling, capping and oxidation, then treating a synthetic plate with a DEA-ACN mixed solution and ACN in sequence, then carrying out ammonolysis, and after the ammonolysis is finished, eluting a sample in the synthetic plate till a brand new 96-hole plate, and obtaining the probe through HPLC purification and separation. According to the method, the step of capping is added before the first-time deprotection in the four-step circular reaction of deprotection, coupling, capping and oxidation of a traditional solid-phase phosphoramidite triester method so as to prevent CPG which is not marked with quenching groups from participating in the subsequent reaction, and the existence of by-products with fluorescence labels is avoided, so that the fluorescence background of the Taqman probe is reduced.
Owner:通用生物(安徽)股份有限公司
Chiral phosphoramides, chiral N-phosphonimines and methods for forming the same
The invention relates free NH2-group-attached chiral phosphoramides, a chiral N-phosphonimines and the methods for forming the same. The free NH2-group-attached chiral phosphoramides, having the structure of formula (I) wherein R1 and R2 are independently any organic groups.
Owner:NOWA PHARMA
Nanometer cyclophosphamide and preparation method thereof
ActiveCN102961343ABioavailability leapEasy to prepareOrganic active ingredientsPharmaceutical non-active ingredientsSide effectTherapeutic effect
The invention discloses nanometer cyclophosphamide and a preparation method thereof, which relate to cyclophosphamide, an antitumor drug. For solving the problem that cyclophosphamide is difficult to dissolve in water, poor in oral absorption and low in bioavailability, and reducing the side effects of cyclophosphamide and improving the therapeutic effect of cyclophosphamide, the invention firstly aims to provide a novel nanometer cyclophosphamide particle, which is characterized by taking silica aerogel as a carrier of cyclophosphamide; and the other purpose of the invention is to provide a method for preparing the nanometer cyclophosphamide particle, which is characterized by comprising the steps of dissolving cyclophosphamide into anhydrous ethanol firstly; then adding the silica aerogel in proportion; after the obtained product is completely adsorbed, drying the obtained object, and adding pure water into the obtained object; and feeding the obtained mixture into an emulsifying machine to emulsify, homogenizing the emulsified product by using a high-pressure homogenizer, and drying the obtained homogenized liquid so as to obtain the nanometer cyclophosphamide particle. The novel nanometer cyclophosphamide disclosed by the invention is especially suitable for oral administration.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com