Sulfur octyl phosphoramido ester and synthetic method and application thereof
A technology of lipoyl ester and phosphoramidite, which is applied in the field of phosphoramidite lipoyl ester and its synthesis, can solve the problems of not being able to meet the needs of scientific research and business, and the lack of disulfide bond-containing terminal reagents, etc., and achieve good results. The effect of commercialization prospects, simplified synthesis workload, good universality and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] A method for preparing the above-mentioned phosphoramidite lipoyl ester, comprising the steps of obtaining lipoic acid through reduction reaction to obtain lipoyl alcohol, and then obtaining phosphoramidite lipoyl ester through esterification.
[0038] The preparation method of concrete above-mentioned phosphoramidite lipoyl ester, its reaction process is as shown in reaction formula I:
[0039]
[0040] Reaction I
[0041] Wherein compound II is lipoic acid, compound III is lipoyl alcohol, and compound I is phosphoramidite lipoyl ester.
[0042] In an optional embodiment: the lipoic acid is prepared in anhydrous tetrahydrofuran using sodium trimethoxyborohydride and boric acid as reducing agents to prepare lipoic alcohol.
[0043] In an optional embodiment: the reduction reaction specifically includes the following steps: dissolving sodium trimethoxyborohydride and boric acid in anhydrous tetrahydrofuran, adding lipoic acid while stirring in an ice bath, and stirri...
Embodiment 1
[0059] A kind of synthetic method of phosphoramidite lipoyl ester, comprises the following steps:
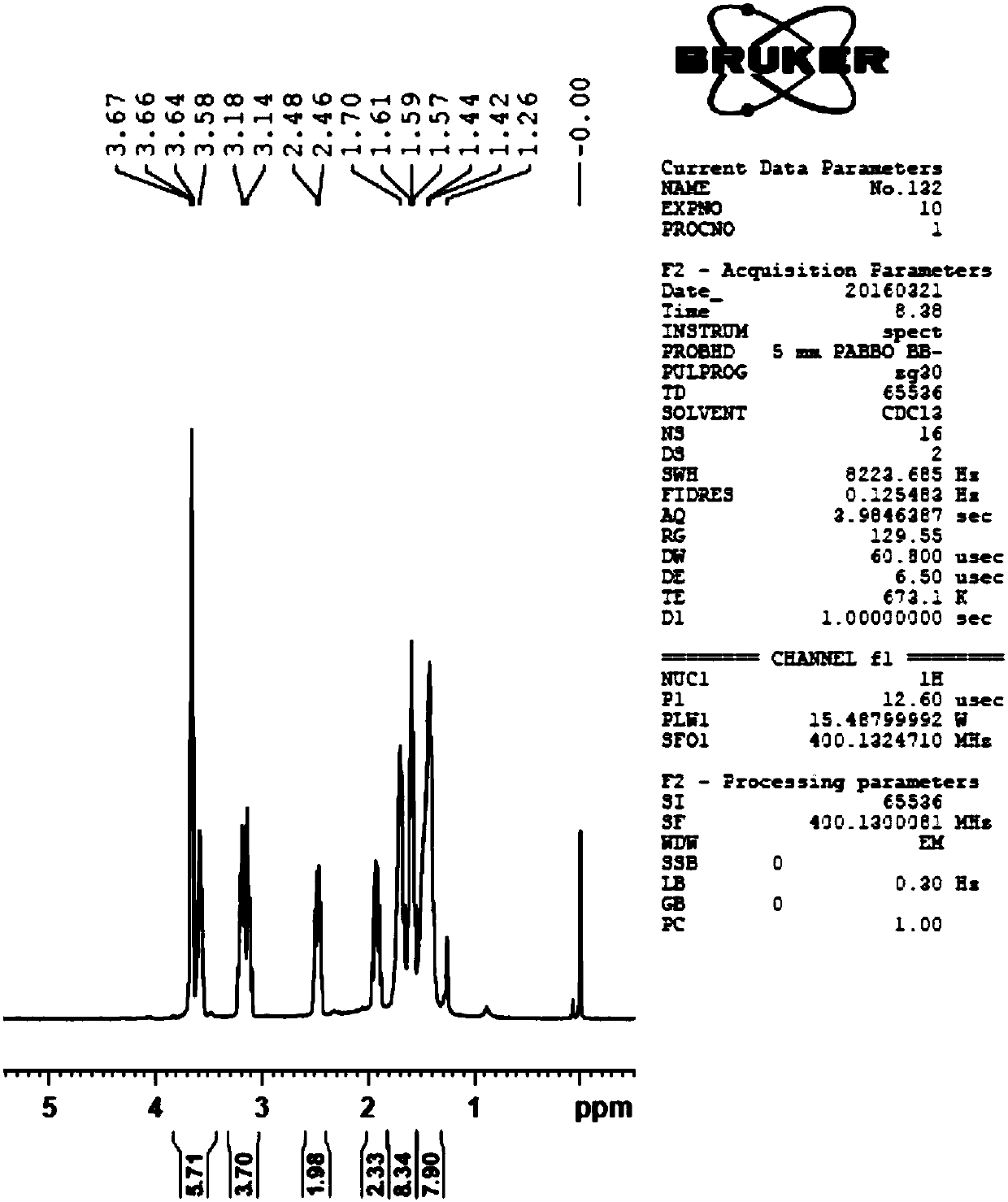
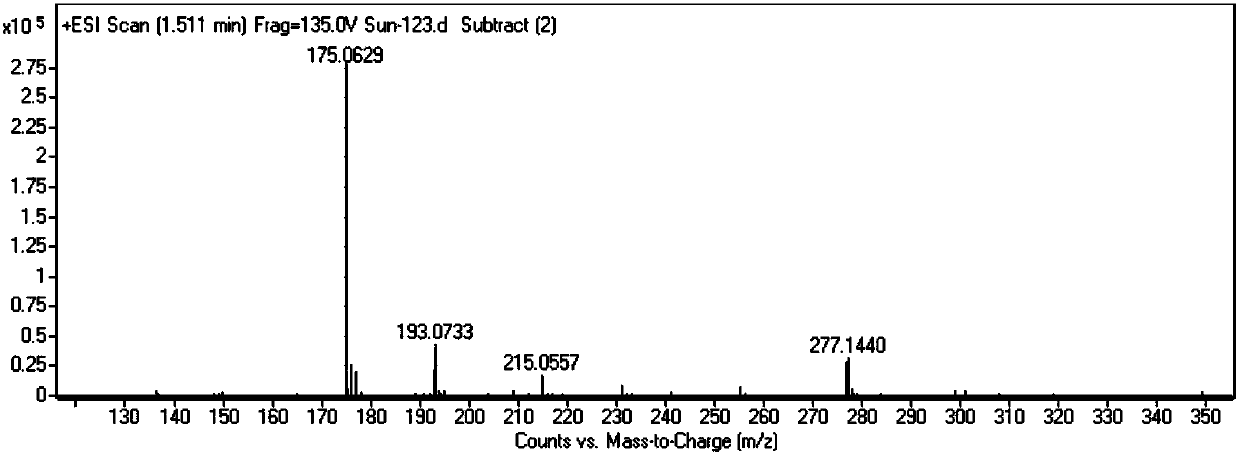
[0060] Step 1: Preparation of lipoic alcohol: Dissolve 1.0 g of sodium trimethoxyborohydride and 0.15 g of boric acid in 66 ml of anhydrous tetrahydrofuran, and add a solution of 1.0 g of lipoic acid in 66 ml of anhydrous tetrahydrofuran at one time while stirring in an ice bath , and the reaction solution was stirred at 15° C. for 30 hours. After the reaction was completed, 9 ml of 3M sulfuric acid solution was added dropwise to the reaction solution, and the insoluble matter was filtered off. The filtrate was washed with 100 ml of chloroform and 100 ml of saturated brine, and the organic phase was separated and dried over anhydrous sodium sulfate. The organic phase was rotary evaporated to obtain 1.5 g of lipoctyl alcohol (pale yellow viscous liquid), with a yield of 81%.
[0061] The proton nuclear magnetic resonance spectrum detection result of compound IV in the intermedia...
Embodiment 2
[0073] A kind of synthetic method of phosphoramidite lipoyl ester, comprises the following steps:
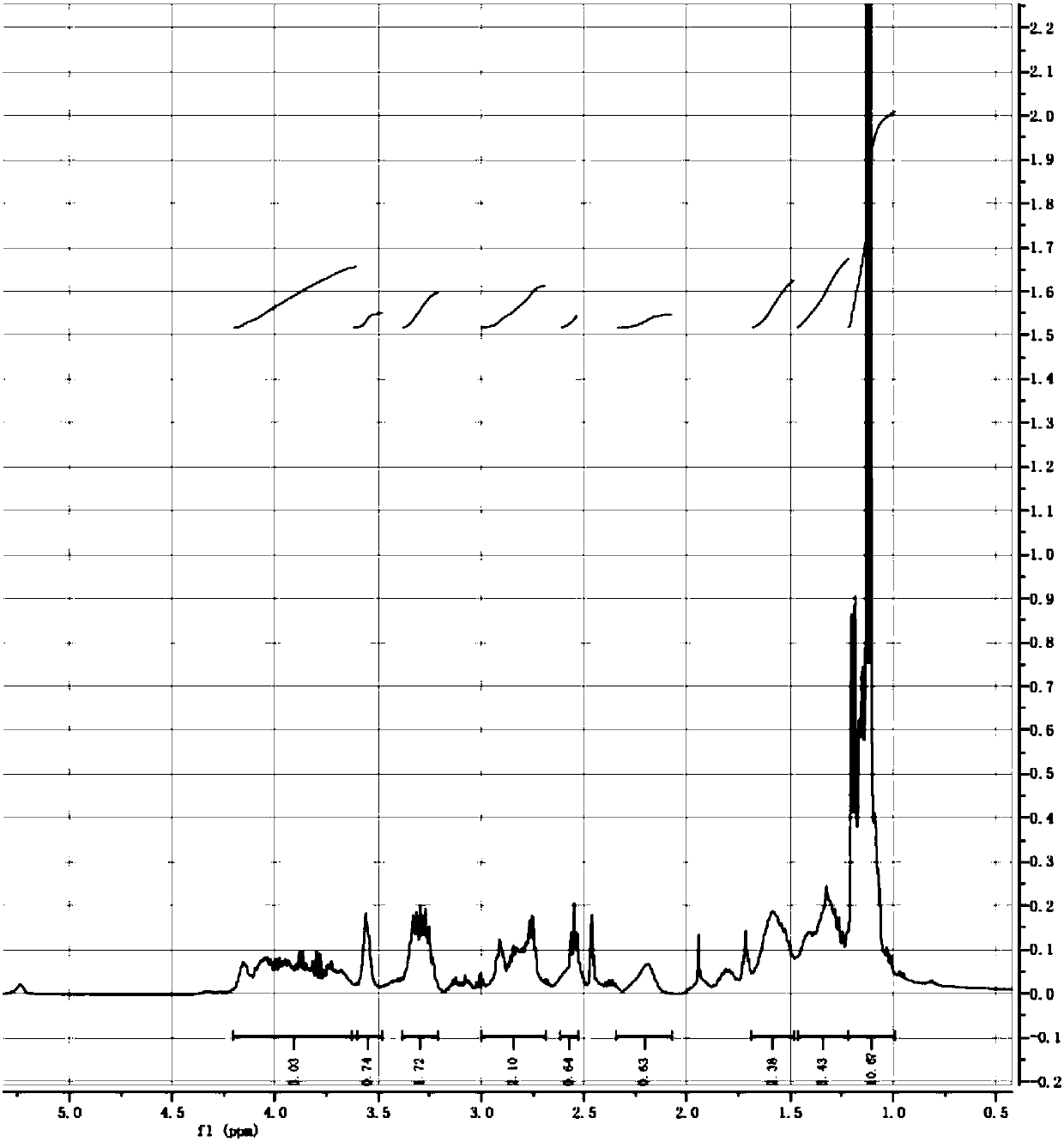
[0074] Step 1: Preparation of lipoic alcohol: Dissolve 3.0 g of sodium trimethoxyborohydride and 0.2 g of boric acid in 200 ml of anhydrous tetrahydrofuran, and add a solution of 2.0 g of lipoic acid in 200 ml of anhydrous tetrahydrofuran at one time while stirring in an ice bath , and the reaction solution was stirred at 20° C. for 24 hours. After the reaction, 20 ml of 4M sulfuric acid solution was added dropwise to the reaction solution, and the insoluble matter was filtered off. The filtrate was washed with 200 ml of chloroform and 100 ml of saturated brine, and the organic phase was separated and dried over anhydrous sodium sulfate. The organic phase was rotary evaporated to obtain 1.6 g of lipoyl alcohol (pale yellow viscous liquid), with a yield of 83%.
[0075] The proton nuclear magnetic resonance spectrum detection result of compound IV in the intermediate product thi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com