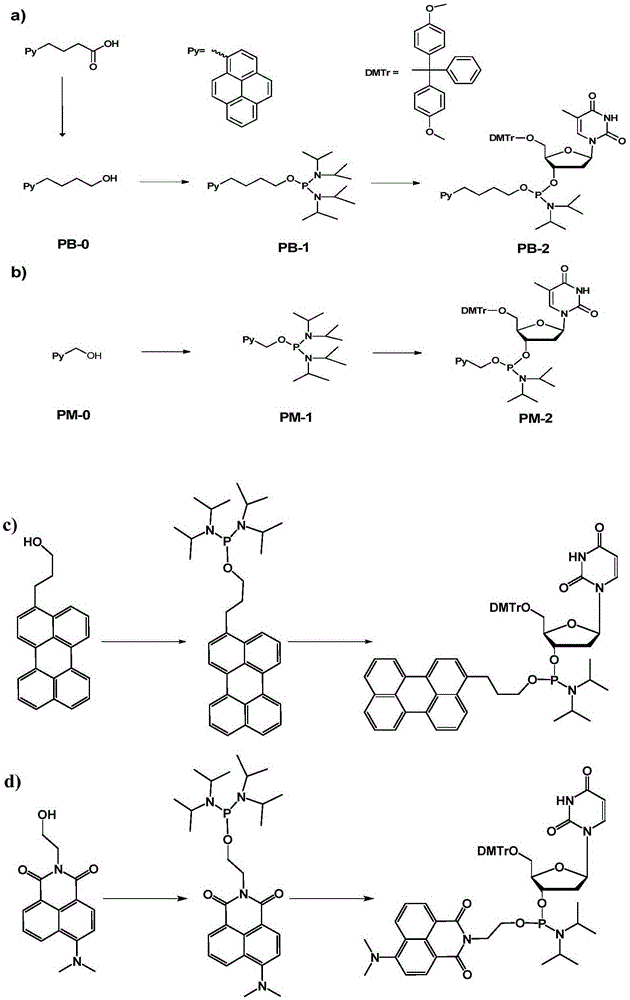
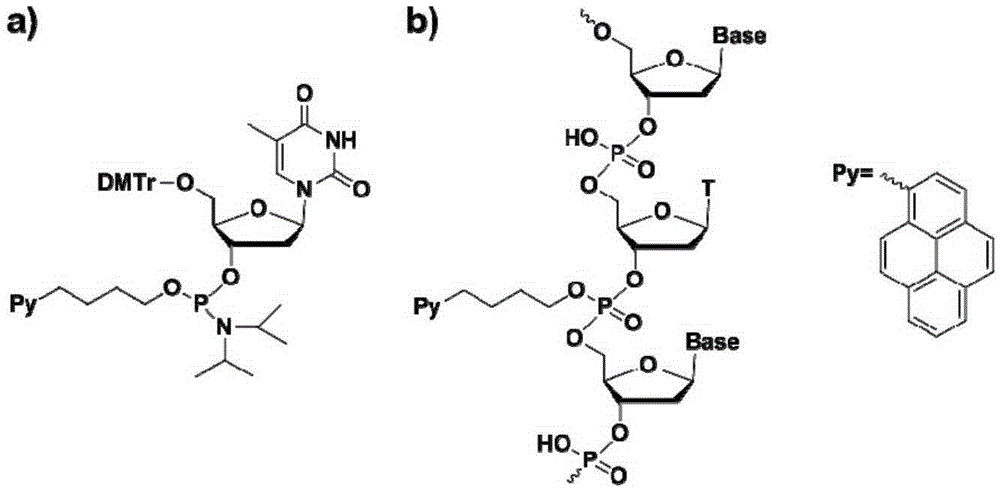
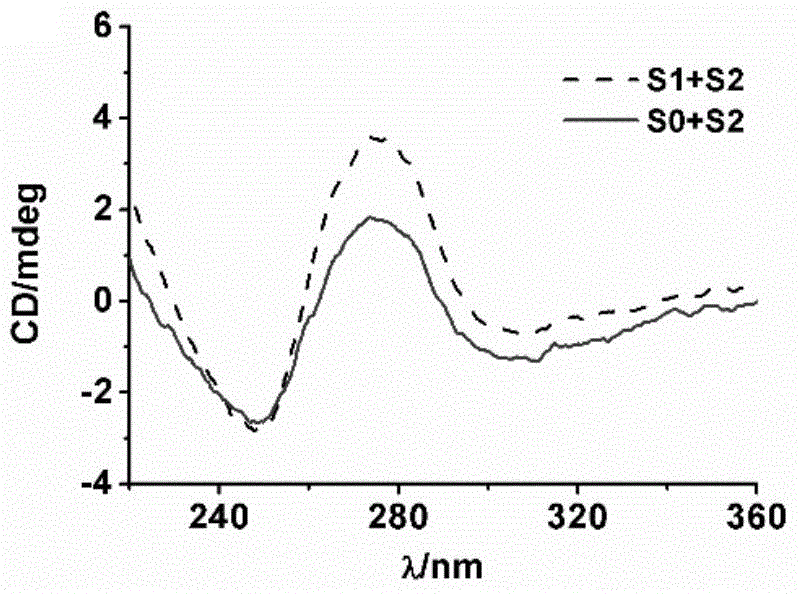
Chromophore-modified deoxynucleoside phosphoramidite monomer compound, preparation method therefor and application thereof
A technology of deoxynucleoside phosphoramidite and deoxynucleoside, which is applied to chromophore-modified deoxynucleoside phosphoramidite monomer compounds and the fields of preparation and application thereof, and can solve the problem that single-base mutation cannot be effectively distinguished, and there is no single-base mutation. Generality, tedious chemical synthesis of pyrene-modified oligonucleotides, etc., to achieve the effects of excellent properties, significant fluorescence enhancement, and strong recognition ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Synthesis of embodiment 1 phosphoric acid position pyrene butanol modified phosphoramidite monomer
[0057] 1. Preparation of Compound PB-0
[0058] 2M concentration of borane dimethyl sulfide tetrahydrofuran solution 3mL, ice bath cooled to zero; pyrene butyric acid (560mg, 1.94mmoL, purchased from Bailingwei company) was dissolved in freshly distilled anhydrous THF solution 5mL. Under stirring in an ice bath, the tetrahydrofuran solution of pyrenebutyric acid was added dropwise to the borane solution, and stirred overnight at room temperature. The reaction mixture was stirred in an ice bath, and methanol was added dropwise to quench the reaction until no bubbles were generated, and the stirring was continued for 2 hours. The reaction system was concentrated to a small amount under reduced pressure, and 30 mL of ethyl acetate was added; the mixed solution was washed with saturated sodium carbonate solution (30 mL×3). The organic phase was collected, dried over anhydr...
Embodiment 2
[0064] Synthesis of embodiment 2 phosphoric acid position pyrene methanol modified phosphoramidite monomer
[0065] 1. Preparation of compound PM-1
[0066]Under nitrogen protection, bis-diisopropylphosphorus amidochloride (293mg, 1.1mmoL) was dissolved in 5mL of anhydrous tetrahydrofuran, and 1mL of triethylamine was added, and cooled to 0°C in an ice bath. Pyrenemethanol (230mg, 1.0mmoL) was dissolved in 1mL of anhydrous tetrahydrofuran under nitrogen protection, added dropwise to the former solution, and stirred overnight at room temperature. After the reaction was completed, the insoluble matter was quickly filtered off, and the filtrate was quickly spin-dried to obtain a light yellow solid. It was directly used in the next reaction without further processing. 31 PNMR (162MHz, CDCl 3 )δ=121.8.
[0067] 2. Preparation of Compound PM-2
[0068] Under nitrogen, DMT-protected deoxythymidine (350 mg, 0.64 mmoL) and tetrazolium (80 mg, 1.1 mmoL) were dissolved in 1.5 mL of ...
Embodiment 3
[0070] Synthesis of embodiment 3 phosphoric acid position perylene propanol modified phosphoramidite monomer
[0071] Synthesis of Compound Perylene-1
[0072] Under nitrogen protection, bis-diisopropylphosphorus amidochloride (293mg, 1.1mmoL) was dissolved in 5mL of anhydrous tetrahydrofuran, and 1mL of triethylamine was added, and cooled to 0°C in an ice bath. Perylene propanol (310 mg, 1.0 mmoL) was dissolved in 1 mL of anhydrous tetrahydrofuran under nitrogen protection, added dropwise to the former solution, and stirred overnight at room temperature. After the reaction was completed, the insoluble matter was quickly filtered off, and the filtrate was quickly spin-dried to obtain a yellow solid. It was directly used in the next reaction without further processing.
[0073] Synthesis of Compound Perylene-dC
[0074] Under nitrogen, DMT-protected deoxycytidine (412 mg, 0.65 mmoL) and tetrazolium (80 mg, 1.1 mmoL) were dissolved in 1.5 mL of anhydrous dichloromethane. All...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com