Methyl propenyl phosphoramidite monomer and synthetic method thereof
A technology of methacryl phosphoramidite and synthesis method, which is applied in the field of hydrogel, can solve the problems of difficult purification of chemically cross-linked DNA hydrogel, and achieve the effects of cheap synthetic raw materials, high polymerization efficiency, and high purification efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 Synthesis of methacryl phosphoramidite monomer. Proceed as follows:
[0030] Step 1: Synthesis of intermediate product 2, the route is as follows:
[0031]
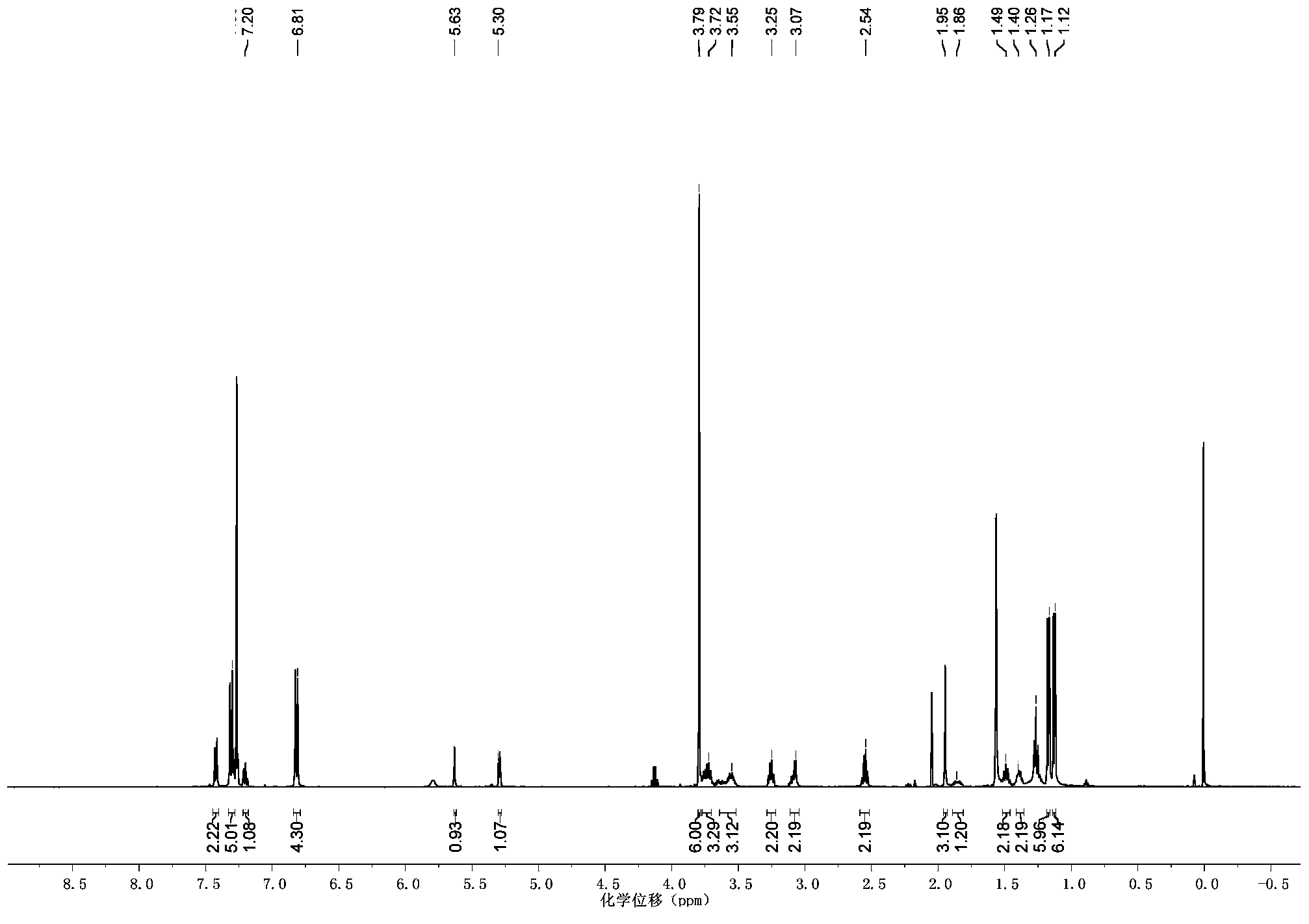
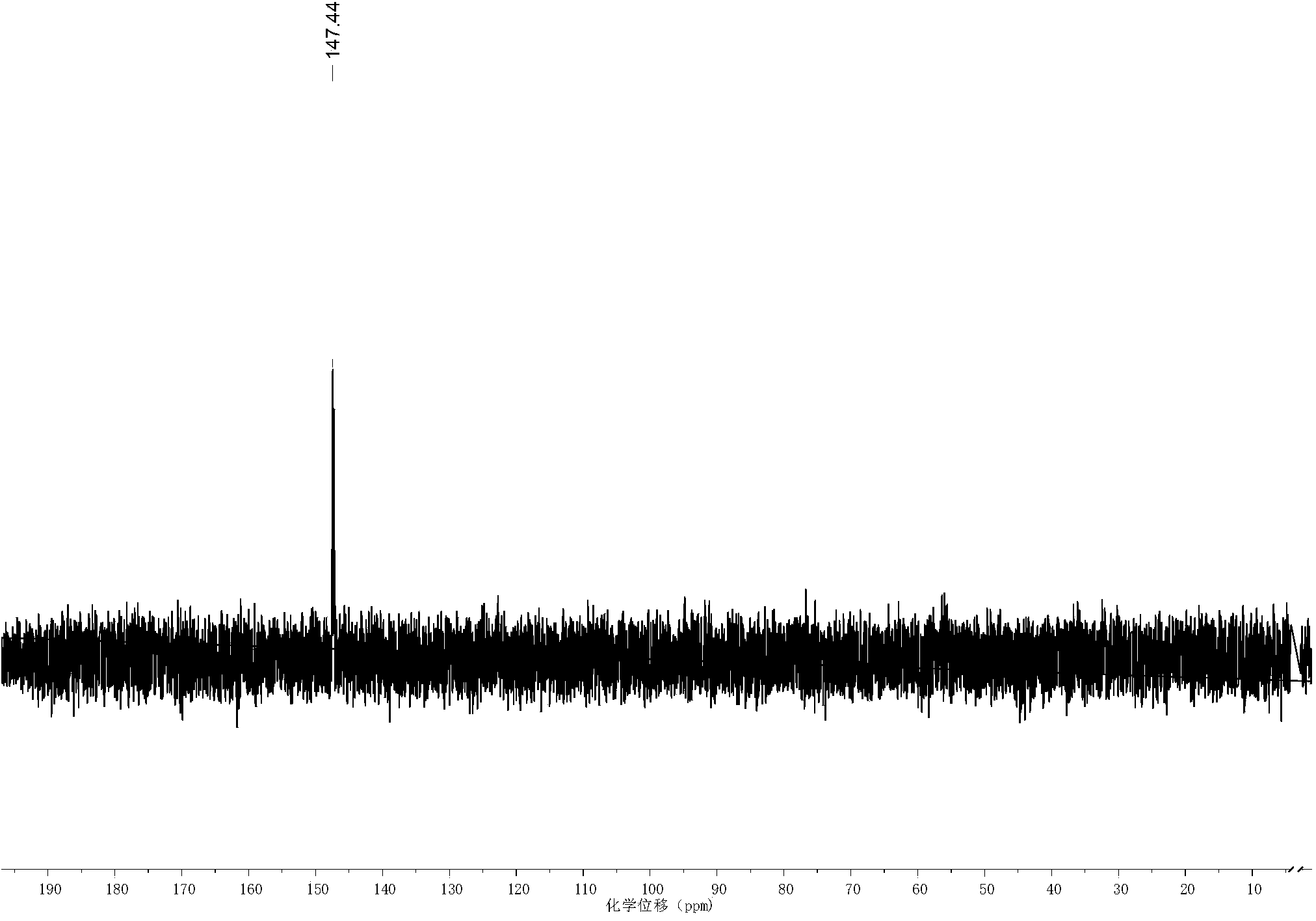
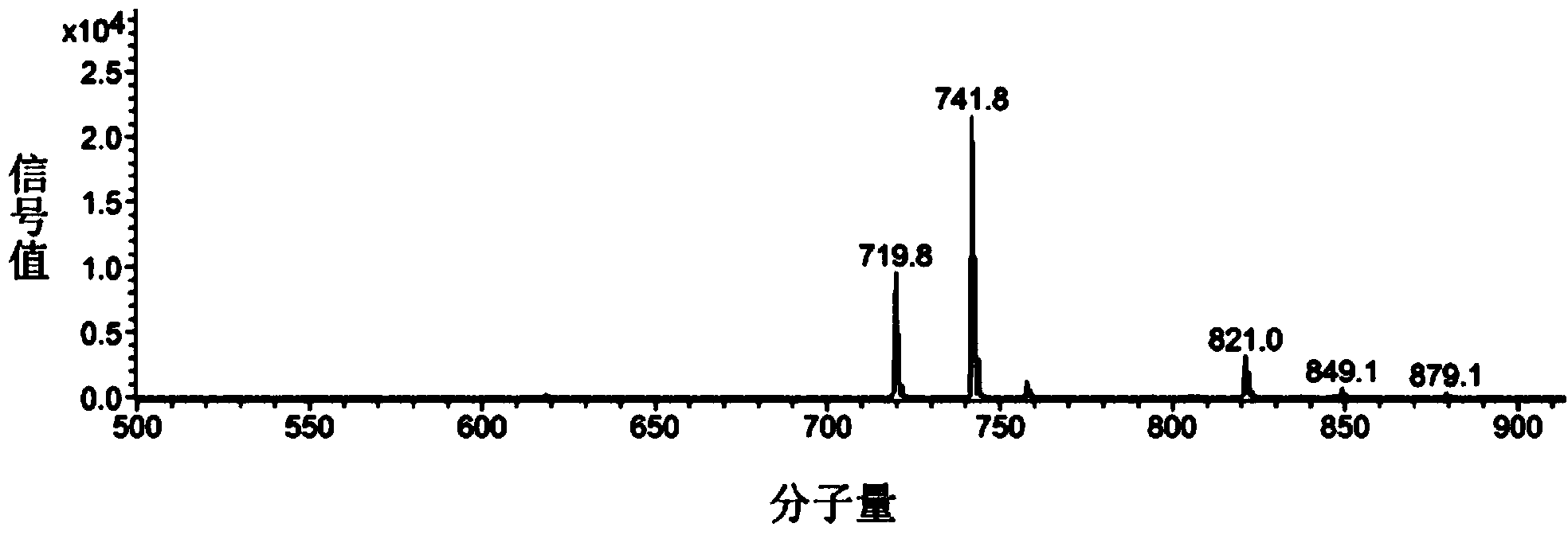
[0032] In a round bottom flask, add methacrylic acid (176mg, 2mmol), 6-Amino-2-hydroxymethyl Hexan-1-ol (294mg, 2mmol), DCC (542.4mg, 2.4mmol), HOBt (324mg, 2.4mmol) , 10mL solvent, under the protection of nitrogen, react at room temperature for 24h, after the end, use silica column to separate and purify, NMR and mass spectrum characterization. 1 H NMR (500MHz, CDCl 3 )δ5.67(s,1H),5.32(s,1H),3.78(q,2H),3.67(q,2H),3.35(q,4H),1.96(s,3H),1.70(m,1H ),1.56(m,2H),1.37(m,4H).ESI-MS Calculated for C 11 h 21 NO 3 Na:238.29([M+Na] + ), Found: 238.7.
[0033] Step 2: Synthesis of intermediate product 3, the synthetic route is as follows:
[0034]
[0035]In a round bottom flask was added 2 (270mg, 1.17mmol), 4-dimethylaminopyridine (14mg, 0.117mmol), 7mL pyridine, under nitrogen protection. At th...
Embodiment 2
[0039] Example 2 Synthesis and purification of nucleic acid molecules modified with methacrylic groups.
[0040] Using ordinary CPG as a solid phase carrier, using DNA monomer bases as raw materials, synthesize DNA sequence strand A from the 3' end to the 5' end on a DNA synthesizer, and finally modify the methacrylphosphoramidite monomer at the 5' end body. The specific synthetic sequence is as follows: 5'-X AAA ATC ACA GAT GAG T-3', wherein x is the product 4. After the synthesis, transfer the above CPG to a 2ml clean and sterilized Eppendorf tube, add 0.4mL of methylamine:ammonia water=1:1 solution, ammonolysis at 65°C for 30min, and cut the DNA from the CPG. After ammonium hydrolysis, extract the supernatant, wash the CPG with a small amount of ultrapure water, and combine the supernatant. Add 2.5 times the volume of frozen absolute ethanol and 0.1 times the volume of 3mol / L NaCl to the system, and carry out alcohol precipitation in a -20°C refrigerator. After the ethan...
Embodiment 3
[0041] Example 3 Preparation of linear high molecular weight DNA polymer.
[0042] Dissolve the isolated and purified strand A in ultrapure water to prepare a DNA aqueous solution. Prepare 10% ammonium persulfate and 5% N,N,N,N-tetramethylethylenediamine (TEMED), respectively, that is, dissolve 0.05g ammonium persulfate into 0.5ml ultrapure water and 25μl TEMED into 0.5ml Ultra-pure water. Prepare a mixture of DNA with a final concentration of 1 mM and acrylamide with a final concentration of 4%, and put it in a vacuum desiccator to vacuum and degas for 10 min. Add freshly prepared initiator (ammonium persulfate) and accelerator (TEMED) with a final concentration of 1.4%, mix well, place the reaction system in a vacuum desiccator, and react under vacuum for 15 minutes at room temperature to obtain the strand A linear High molecular DNA polymer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com