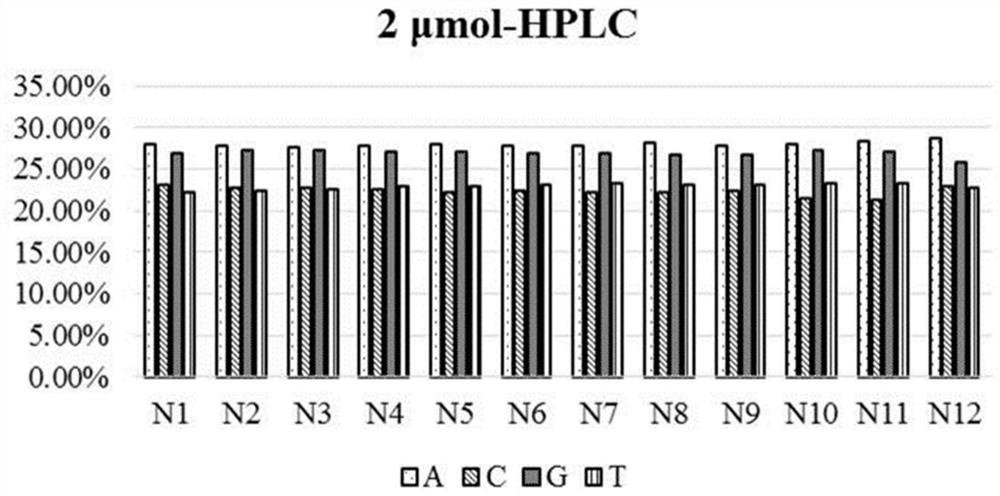
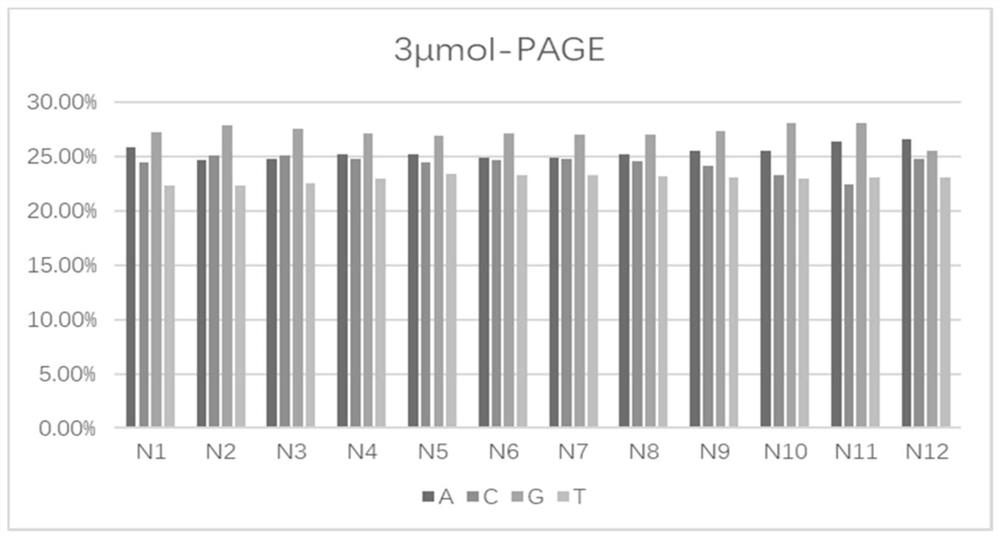
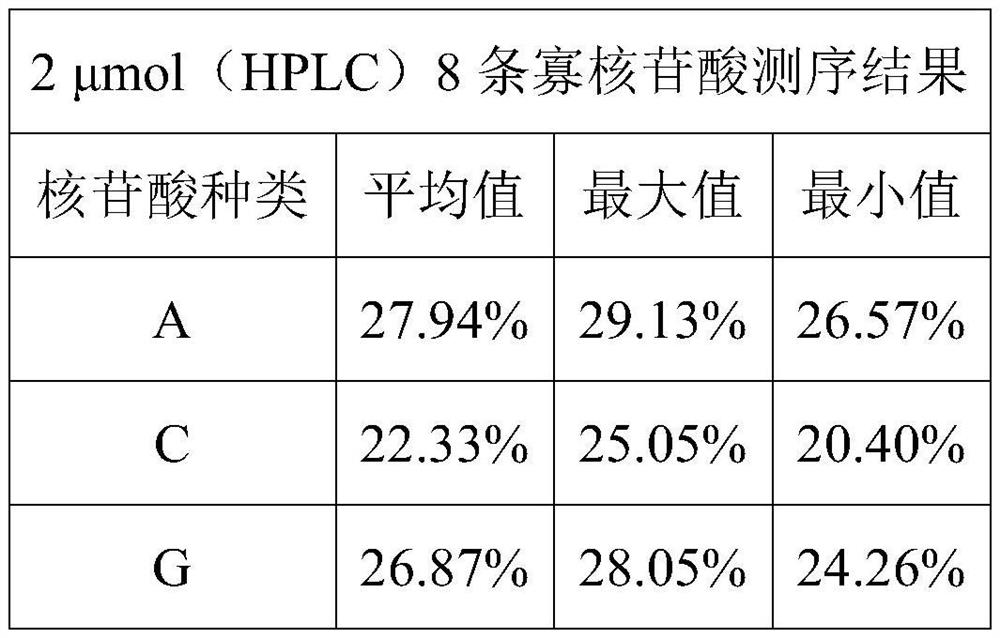
Phosphoramidite monomer composition and preparation method and application thereof
A technology of monomer composition and phosphoramidite, which is applied to the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problem of lack of convincingness, inability to directly characterize the degeneracy of synthetic sequences, and inability to directly "read" "Oligo and other problems have been solved to achieve the effect of strong practicability and repeatability, and intuitive and effective detection results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] This embodiment prepares phosphoramidite monomer composition, comprises the following steps:
[0050] (1) 10g of 5'-O-(4,4'-dimethoxytriphenyl)-N6-benzoyl-2'-deoxyadenosine-3'-(2-cyanoethyl-N, N-diisopropyl) phosphoramidite (abbreviated as dA monomer) is dissolved in 333mL acetonitrile;
[0051] 10g of 5'-O-(4,4'-dimethoxytriphenyl)-N4-acetyl-2'-deoxycytidine-3'-(2-cyanoethyl-N,N-diiso Propyl) phosphoramidite (abbreviated as dC monomer) was dissolved in 370mL acetonitrile;
[0052] 10g of 5'-O-(4,4'-dimethoxytriphenyl)-N2-(N,N-dimethylformamidine)-2'-deoxyguanosine-3'-(2-cyano Ethyl-N,N-diisopropyl) phosphoramidite (abbreviated as dG monomer) was dissolved in 340mL acetonitrile;
[0053] 10g of 5'-O-(4,4'-dimethoxytriphenyl)-2'-deoxythymidine-3'-(2-cyanoethyl-N,N-diisopropyl)phosphite Amide (abbreviated as dT monomer) was dissolved in 383mL acetonitrile;
[0054] (2) Add dA solution, dC solution, dG solution and dT solution into the dry reagent bottle according to ...
Embodiment 2
[0056] This embodiment prepares phosphoramidite monomer composition, comprises the following steps:
[0057] (1) 10g of 5'-O-(4,4'-dimethoxytriphenyl)-N6-benzoyl-2'-deoxyadenosine-3'-(2-cyanoethyl-N, N-diisopropyl) phosphoramidite (abbreviated as dA monomer) is dissolved in 400mL acetonitrile;
[0058] 10g of 5'-O-(4,4'-dimethoxytriphenyl)-N4-acetyl-2'-deoxycytidine-3'-(2-cyanoethyl-N,N-diiso Propyl) phosphoramidite (abbreviated as dC monomer) was dissolved in 400mL acetonitrile;
[0059] 10g of 5'-O-(4,4'-dimethoxytriphenyl)-N2-(N,N-dimethylformamidine)-2'-deoxyguanosine-3'-(2-cyano Ethyl-N,N-diisopropyl)phosphoramidite (abbreviated as dG monomer) was dissolved in 400mL acetonitrile;
[0060] 10g of 5'-O-(4,4'-dimethoxytriphenyl)-2'-deoxythymidine-3'-(2-cyanoethyl-N,N-diisopropyl)phosphite Amide (abbreviated as dT monomer) was dissolved in 400mL acetonitrile;
[0061] (2) Add dA solution, dC solution, dG solution and dT solution into the dry reagent bottle according to t...
Embodiment 3
[0063] This embodiment prepares phosphoramidite monomer composition, comprises the following steps:
[0064] (1) 10g of 5'-O-(4,4'-dimethoxytriphenyl)-N6-benzoyl-2'-deoxyadenosine-3'-(2-cyanoethyl-N, N-diisopropyl) phosphoramidite (abbreviated as dA monomer) was dissolved in 285.7mL acetonitrile;
[0065] 10g of 5'-O-(4,4'-dimethoxytriphenyl)-N4-acetyl-2'-deoxycytidine-3'-(2-cyanoethyl-N,N-diiso Propyl) phosphoramidite (abbreviated as dC monomer) was dissolved in 333mL acetonitrile;
[0066] 10g of 5'-O-(4,4'-dimethoxytriphenyl)-N2-(N,N-dimethylformamidine)-2'-deoxyguanosine-3'-(2-cyano Ethyl-N,N-diisopropyl) phosphoramidite (referred to as dG monomer) was dissolved in 333mL acetonitrile;
[0067] 10g of 5'-O-(4,4'-dimethoxytriphenyl)-2'-deoxythymidine-3'-(2-cyanoethyl-N,N-diisopropyl)phosphite Amide (abbreviated as dT monomer) was dissolved in 333mL acetonitrile;
[0068] (2) Add dA solution, dC solution, dG solution and dT solution into the dry reagent bottle according ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com