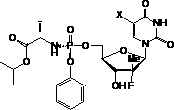
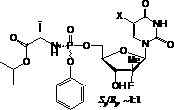
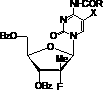
Nucleoside phosphamide prodrug as well as preparation method and application of nucleoside phosphamide prodrug
A technology of coupling reaction and reaction temperature, applied in the field of compounds, can solve problems such as difficult treatment of HCV-I virus infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133] Example 1 3',5'- O -Dibenzoyl-2'-deoxy-2'-fluoro-2'- C -Methyl-N 4 -Preparation of p-benzoyl-5-fluorocytidine
[0134] 1) Add 49g of trifluoroethanol dropwise to 240ml of toluene and 150g of 70% reduced aluminum (Red-Al) / toluene mixed solution under anhydrous, anaerobic, -15°C and stirring conditions. Stir at room temperature for 0.5h to prepare reduced aluminum (Red-Al(OCH 2 CF 3 )) solution;
[0135] 2) Under anhydrous, anaerobic, -10°C and stirring conditions, add dropwise the trifluoroethanol-modified reduced aluminum (Red-Al(OCH 2 CF 3 )) solution; after the dropwise addition, continue to stir at -10°C for 0.5h, and TLC detects that the reaction is complete;
[0136] 3) Maintain anhydrous, anaerobic, -10 ℃, stirring conditions, after adding 12g of tetrabutylammonium bromide, dropwise add 138g of sulfuryl chloride (SO 2 Cl 2 ), after the dropwise addition was completed, stir at room temperature for 16 h, after TLC de...
Embodiment 2
[0143] Example 2 3',5'- O -Dibenzoyl-2'-deoxy-2'-fluoro-2'- C - Preparation of methyl-5-fluorouridine
[0144] Weigh the 3', 5'- prepared in Example 1 O -Dibenzoyl-2'-deoxy-2'-fluoro-2'- C -Methyl-N 4 - 100 grams of p-benzoyl-5-fluorocytidine, put it into 1.5L of 70% acetic acid aqueous solution, stir, heat to reflux for 20h, after TLC detects that the reaction is complete, cool to room temperature, add 400ml of water, and stir at room temperature 2h, suction filtration under reduced pressure, the filter cake was washed with water and dried to obtain 3',5'- O -Dibenzoyl-2'-deoxy-2'-fluoro-2'- C -Methyl-5-fluorouridine 72g, in the form of off-white solid.
[0145] δ( 1 HNMR, DMSO- d 6 ): 1.50 (d, J =22.4Hz, 3H), 4.59(m, 2H), 5.05(m, 1H), 5.95(m, 1H), 6.47(d, J =20.0Hz, 1H), 7.45(m, 2H), 7.57(m, 2H), 7.60(m, 1H), 7.71(m, 1H), 7.92(m, 2H), 8.01(m, 2H), 8.01 (d, J=7.8Hz, 1H), 11.40 (s, 1H) ppm; δ( 19 FNMR, DMSO- d 6 ): -167.8(s), -172.2(...
Embodiment 3
[0146] Example 3 3',5'- O -Dibenzoyl-2'-deoxy-2'-fluoro-2'- C - Preparation of methyl-5-fluorouridine
[0147] 1) Add 8.16g of trifluoroethanol dropwise to 40ml of toluene and 25g of 70% reduced aluminum (Red-Al) / toluene mixed solution under anhydrous, anaerobic, -15°C and stirring conditions. After the dropwise addition, Stir at room temperature for 0.5h to obtain trifluoroethanol-modified reduced aluminum (Red-Al(OCH 2 CF 3 )) solution;
[0148] 2) Add the trifluoroethanol-modified reduced aluminum (Red-Al(OCH 2 CF 3 )) solution; after the dropwise addition, continue to stir at -10°C for 0.5h, and TLC detects that the reaction is complete;
[0149] 3) Maintain anhydrous, anaerobic, -10°C, stirring conditions, add 2g of tetrabutylammonium bromide, drop 23g of SO 2 Cl 2 ;
[0150] 4) After the dropwise addition, stir at room temperature for 16 hours. After TLC detects that the reaction is complete, cool the reaction mixture to 0°C, add 200m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com