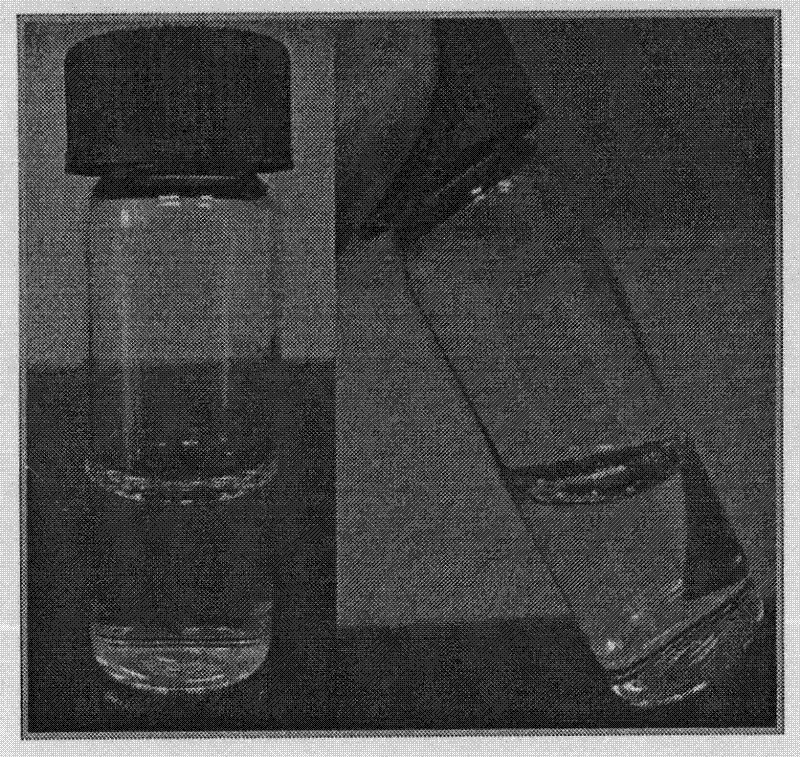Synergistic treatment type multi-material sustained-release eye drop and preparation method
An eye drop, multi-substance technology, applied in the fields of biomaterials and biomedicine, can solve the problems of difficult to maintain controlled release and sustained release of drugs, short administration period, and difficult to control the release rate of drug components, etc. Achieve the effects of excellent drug controlled release, improved drug availability, and excellent biosafety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
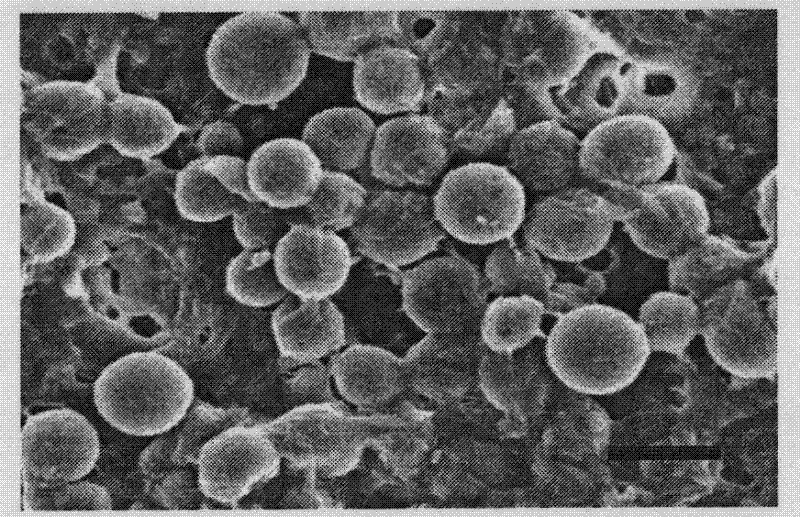
[0032] Weighing average molecular weight is 80000, and the degree of deacetylation 85% chitosan is dissolved in 3g / L acetic acid dilute solution and is mixed with 100 milliliters of chitosan solution of 20g / L, then adds cyclosporine A, cyclosporine A The mass ratio of the chitosan in the solution is 1:8, and it is uniformly mixed and loaded into the syringe of the electrospinning device;
[0033] Dissolve hyaluronic acid in water to prepare 100 ml of hyaluronic acid hydrosol with a concentration of 10g / L, then place it in a container and place it directly below the needle tip of a hollow metal needle of a syringe, and adjust the distance between the needle tip and the liquid level of hyaluronic acid hydrosol. The distance is 9cm, the solution is grounded with a wire, and continuously stirred at 120 rpm, the hollow metal needle is connected to a DC voltage of 10kV, and the chitosan solution in the syringe is pushed out at a speed of 0.20ml / hour for electrostatic spraying, with a...
Embodiment 2
[0035] With the preparation method of embodiment 1, the difference is: to the average molecular weight is 65000, in the chitosan solution of degree of deacetylation 88%, add 1: 1: 1 levofloxacin, natamycin and sodium cromoglycate, control levofloxacin, natamycin The ratio of the total mass of cromolyn sodium to the mass of chitosan in the solution is 1: 3, the DC voltage applied by the metal needle is changed to 18kV, and the distance between the needle tip and the grounded 12g / L hyaluronic acid hydrosol is changed to 18cm, the eye drops containing levofloxacin were prepared by high-voltage electrostatic spraying, and the eye drops attached to the eye drops were photographed by a digital camera. image 3 Shown, show that the eye drops prepared by this method are liquid preparations with good fluidity.
Embodiment 3
[0037] With the preparation method of Example 1, the difference is: the average molecular weight is 50000, and the degree of deacetylation is 82% chitosan solution, adding 1: 3 tobramycin and dexamethasone, and the concentration of the receiving solution is 6.0g / L. Hyaluronic acid hydrosol, the ratio of the total mass of tobramycin and dexamethasone to the mass of chitosan in the solution is controlled to be 1:2, and the gel state loaded with tobramycin and dexamethasone is prepared by 3kV high-voltage electrostatic spraying Eye drops, which are attached to the eye drops when photographed by a digital camera Figure 4 Shown, show that the eye drops prepared by this method are liquid preparations with good fluidity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com