Method for preparing in-situ therapeutic substance-loaded microcapsules
A therapeutic and microcapsule technology, which is applied in the field of biomaterials and biomedicine, can solve the problem that it is difficult to meet the stable and sustained release of microcapsules, the collapse of the microcapsule wall membrane, the controllable release of drugs and the difficulty in maintaining sustained release and other issues, to achieve the effects of excellent biological safety, adjustable particle size, and excellent controlled release of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The weighed average molecular weight is 50000, the degree of deacetylation is 83% chitosan is dissolved in 4g / L acetic acid dilute solution to prepare a 30g / L chitosan solution, then 0.01g cyclosporine A is added, and the Inside the syringe of the electrospinning device;
[0031] Dissolve sodium alginate in water to prepare an aqueous solution of sodium alginate with a concentration of 12 g / L, then place it in a container and place it directly below the needle tip of a hollow metal needle of a syringe, and adjust the distance between the needle tip and the liquid surface of the sodium alginate solution to be 8cm, The solution is grounded with a wire and continuously stirred at 60 rpm. The hollow metal needle is connected to a 10kV DC voltage, and the chitosan solution in the syringe is pushed out at a speed of 0.10 ml / hour for electrostatic spraying, containing cyclosporine A. The chitosan solution is instantaneously formed into a mist of gel particles under the action ...
Embodiment 2
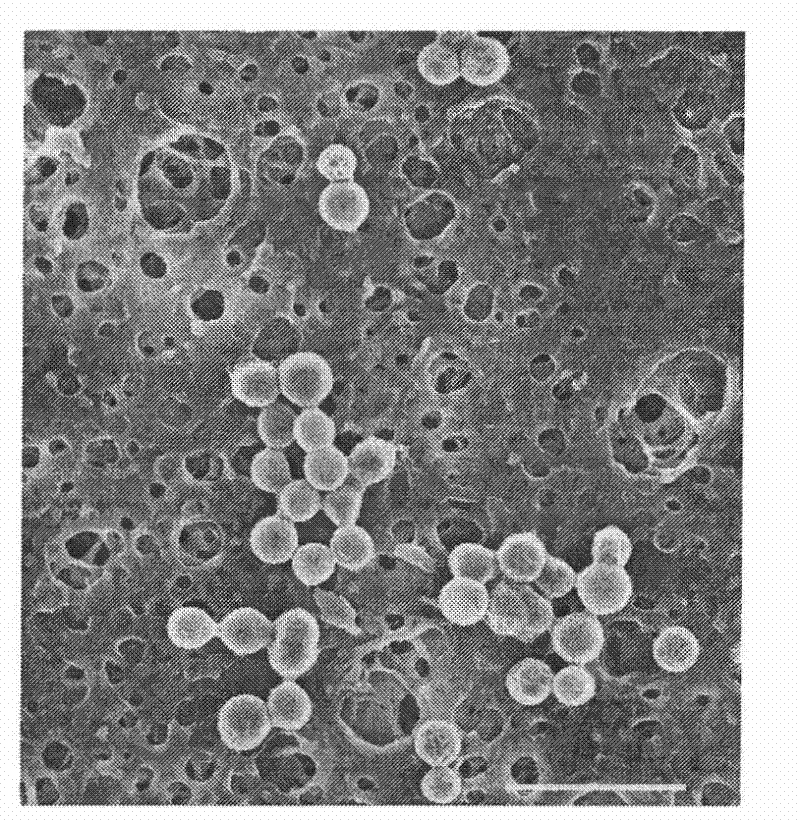
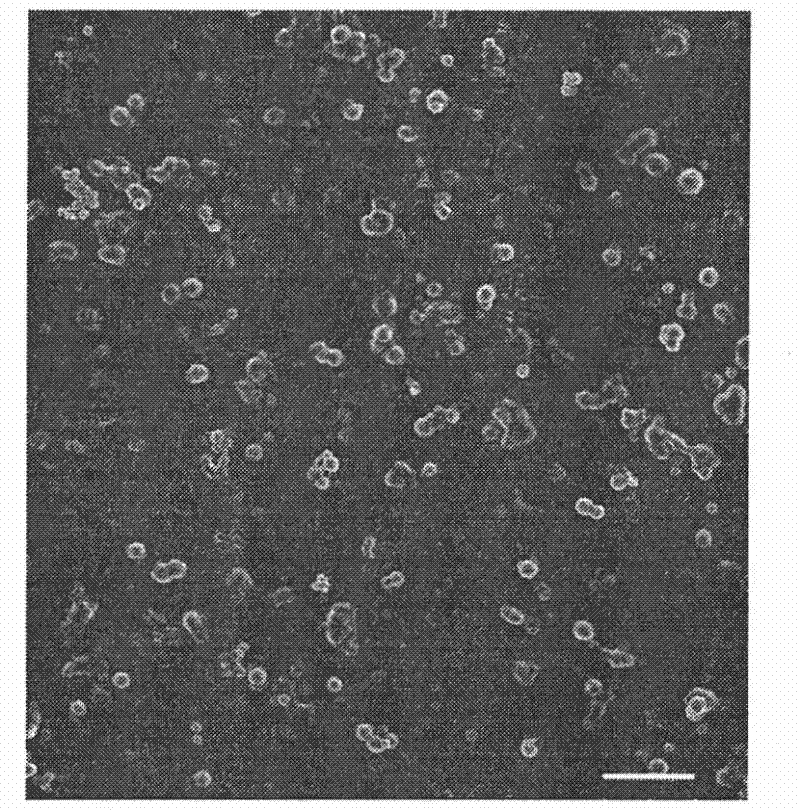
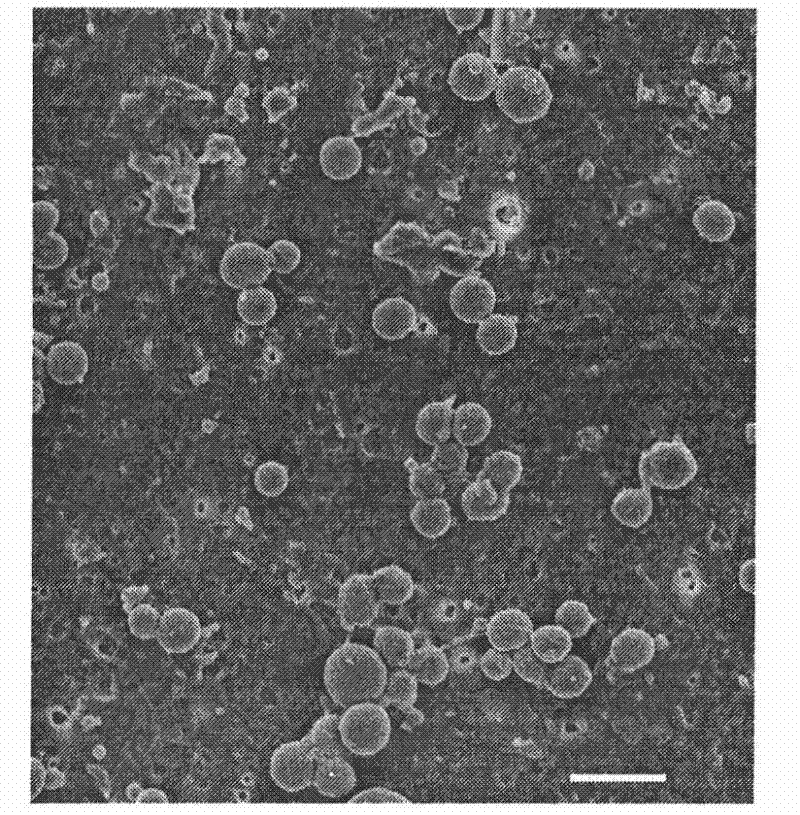
[0033] With the preparation method of Example 1, the difference is: add vascular endothelial growth factor to the chitosan solution, change the DC voltage applied by the metal needle to 15kV, and the distance between the needle tip and the grounded 12g / L sodium alginate receiving solution Change it to 12cm, make microcapsules loaded with vascular endothelial growth factor through high-voltage electrostatic spraying, and observe the morphology of this kind of microcapsules with a scanning electron microscope as attached figure 2 As shown, the microcapsules prepared by this method are nearly spherical nanoparticles.
Embodiment 3
[0035] Sodium alginate was dissolved in water to prepare a sodium alginate aqueous solution with a concentration of 30g / L, and then 0.001g of nano-titanium dioxide with a particle size of 30nm was added, mixed evenly and loaded into the syringe of the electrospinning device;
[0036] Weighing average molecular weight is 250 000, and 86% chitosan of degree of deacetylation is dissolved in 4g / L acetic acid dilute solution and is mixed with the chitosan solution of 1.2g / L, then is placed in the container and placed on the needle point of the hollow metal needle of syringe. Below, adjust the distance between the needle tip and the liquid surface of the chitosan solution to be 10cm, the solution is grounded with a wire, and continuously stirred at 120 rpm, the hollow metal needle is connected to a 15kV DC voltage, and the sodium alginate solution in the syringe is 0.20 ml / min. Push out at the speed of one hour, carry out electrostatic spraying, and the sodium alginate solution conta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com