Peritoneal dialysis medicinal composition with effect of improving side effects
A pharmaceutical composition and peritoneal dialysis technology, applied in the field of renal replacement therapy, can solve the problems of increased permeability, large side effects, loss of ultrafiltration function, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: In vitro culture of human umbilical cord mesenchymal stem cells
[0040] Human umbilical cords were collected aseptically and stored in HBSS (Biochrom L201-10) at 4°C for no more than 24 hours.
[0041] The umbilical cord was first soaked in 75% ethanol for 30 seconds to disinfect. Put the sterilized umbilical cord in a calcium-free and magnesium-free buffer solution (CMF, Gibco 14185-052) in a sterile operating table, cut the umbilical cord longitudinally with sterilized instruments, and cut the blood vessels and mesenchymal tissues ( Watton's gel) removed. The mesenchymal tissue was cut into 0.5 cm3 pieces and centrifuged at 250×g for 5 minutes. The supernatant was removed, and the precipitate was washed twice with an appropriate amount of serum-free DMEM (Gibco 12100-046), and then centrifuged at 250×g for 5 minutes. The mesenchymal tissue was treated with collagenase at 37°C for 14 to 18 hours. After washing, it was treated with 2.5% trypsin at 37°C...
Embodiment 2
[0042] Embodiment 2: In vitro culture of human peritoneal mesothelial cells
[0043] In this experiment, the greater omentum of patients after surgery was treated with 0.125% trypsin and 0.01% EDTA at 37°C for 25 minutes, and then washed with an appropriate amount of 10% FBS DMEM culture medium. skin cells.
Embodiment 3
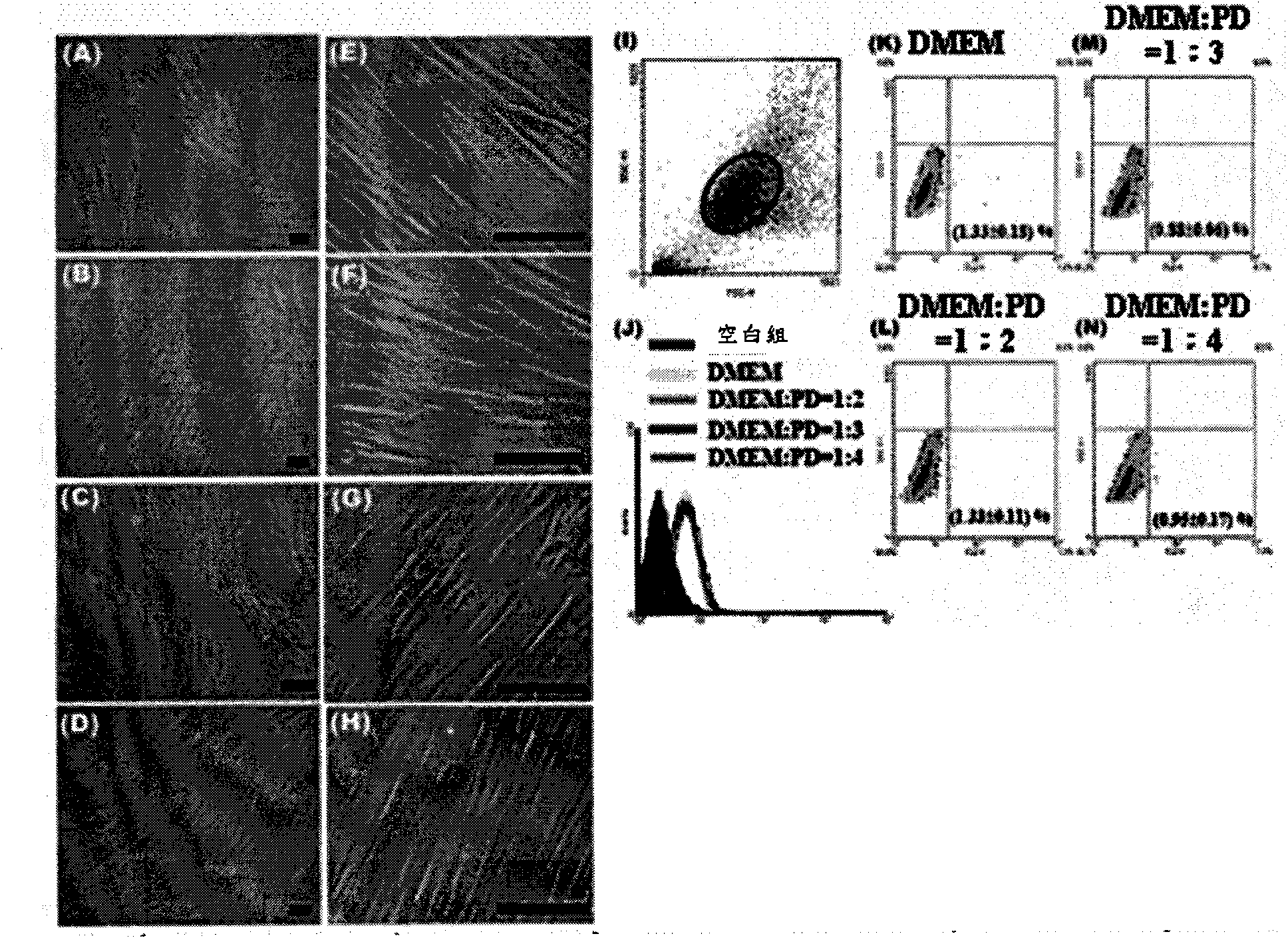
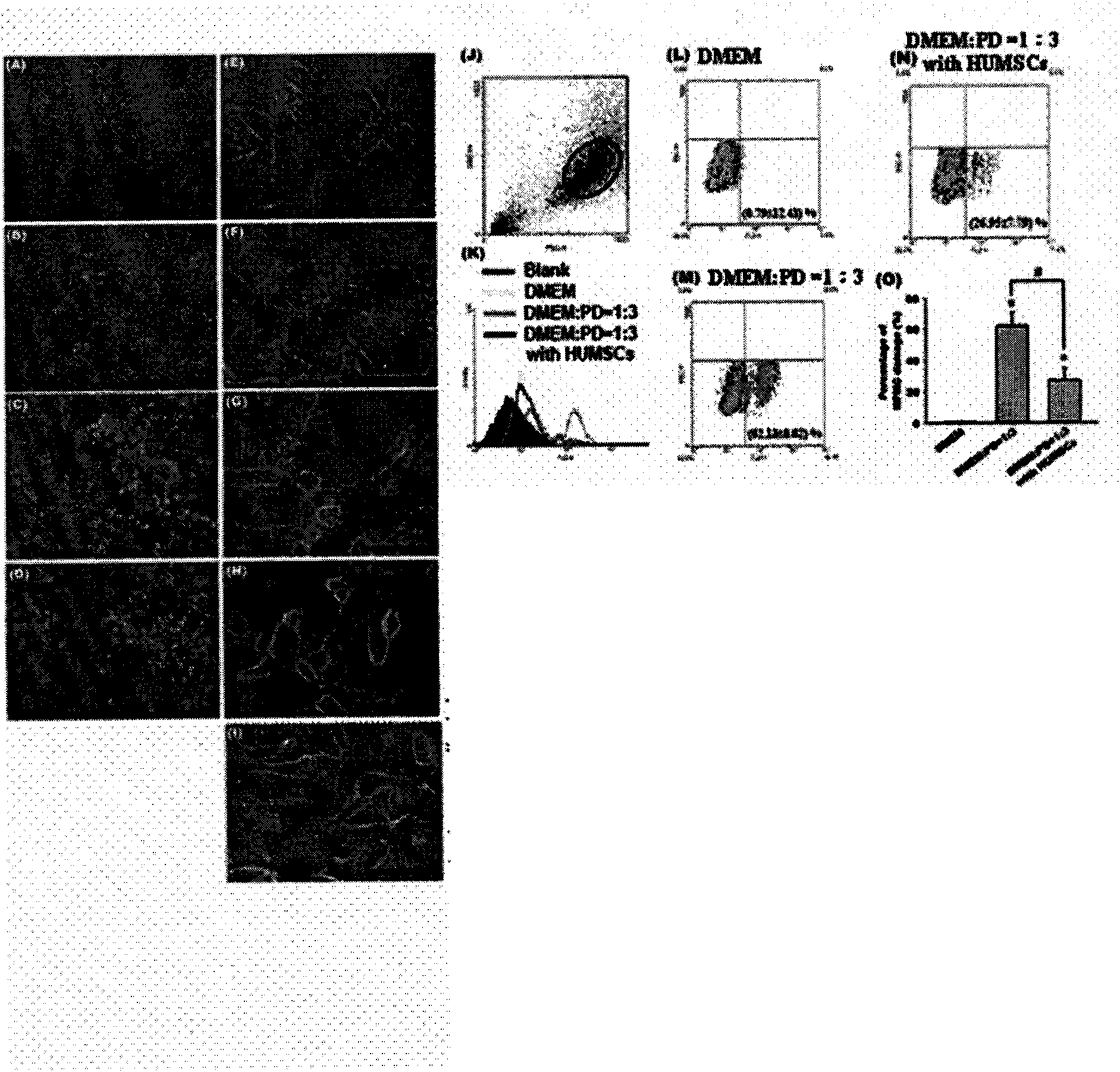
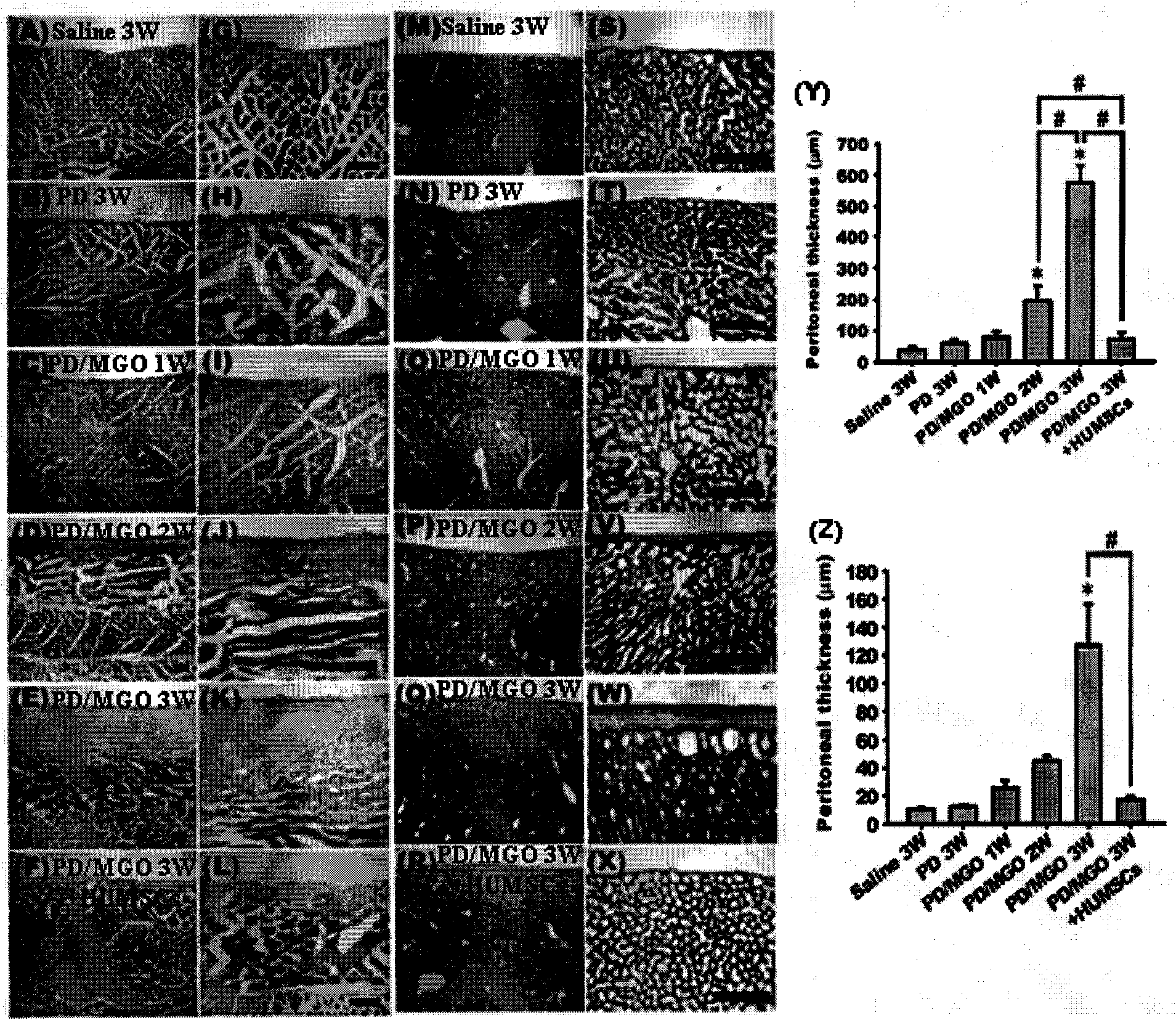
[0044] Example 3: High-glucose dialysate will not cause damage to human umbilical cord mesenchymal stem cells cultured in vitro
[0045] Human umbilical cord mesenchymal stem cells were treated with DMEM medium: PD=1:2, 1:3, and 1:4 for 24 hours respectively, and observed by optical microscope, the cell morphology was similar to that of the control group treated with DMEM ( figure 1 A to 1H). Analysis by flow cytometry also found that umbilical cord mesenchymal stem cells will not be detected to be damaged ( figure 1 1 to 1N).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com