Quinnazolidone derivative, preparation method thereof and purpose of serving as anticarcinogen
A technology of derivatives and quinazolones, which is applied in the field of preparation of anticancer drugs, quinazolones derivatives and their preparations, can solve the problems of less development and research of quinazolones, and achieve normal cell toxicity and broad Application space, good effect of inhibiting activity
Inactive Publication Date: 2010-10-13
SUN YAT SEN UNIV
View PDF2 Cites 9 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Patent application 200810027004 has disclosed a kind of bis-fatty amino substituted quinazolones derivatives and their application as anticancer drugs, however, there are still very few researches on the development of quinazolones at present
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
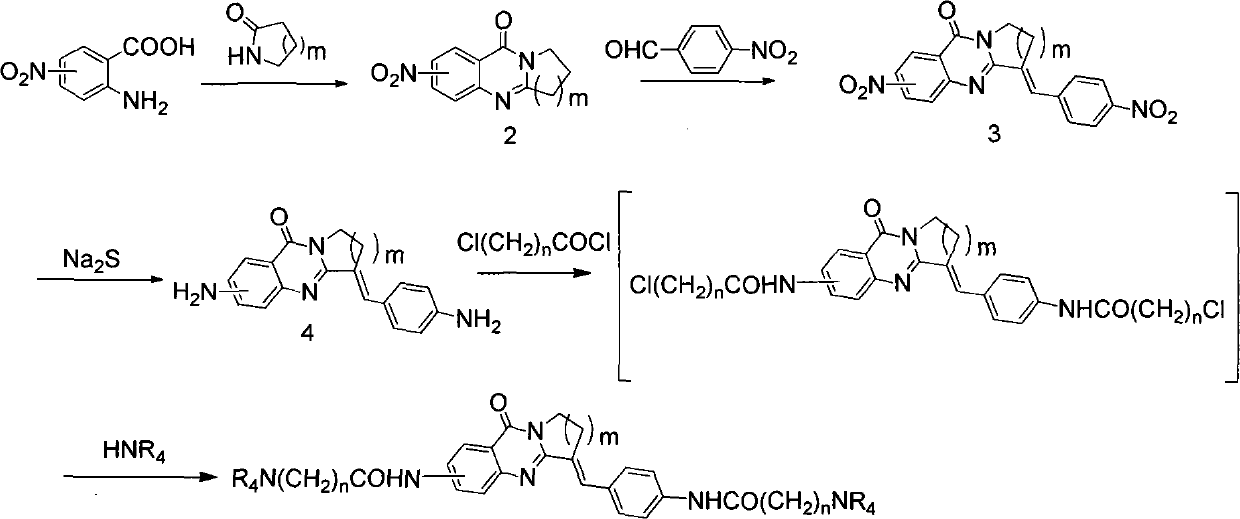
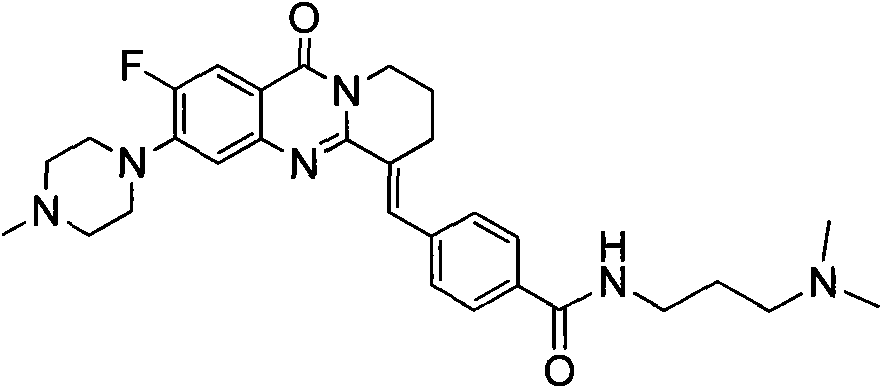
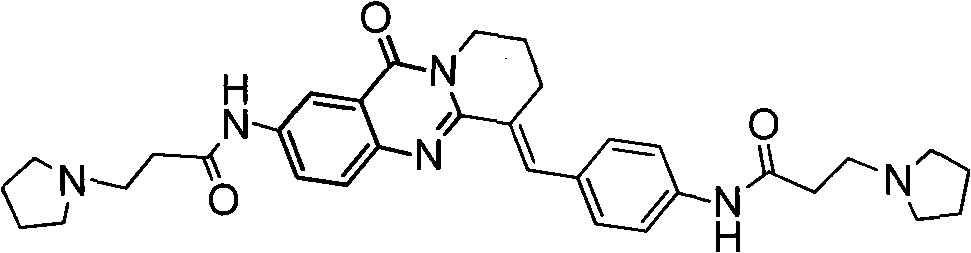
The invention belongs to the medicine and chemical industry field, which discloses a quinnazolidone derivative, a preparation method thereof and a purpose of serving as anticarcinogen. The structural formula of the quinnazolidone derivative is that: R1 is F, CL, Br or I, R2 is NH (CH2) nNR4, NR4 or NH(CH2)n-Ar, R3 is NHCO (CH2) nNR4 or CONH (CH2) nNR4, and m equals to 1,2 or 3. -Ar represents various aromatic nucleus, including various heteroaromatic compounds; n equals to 1, 2, 3, 4 or 5; R4 represents C1-6alkyl, C3-6 naphthenic base, piperidyl, morpholinyl, piperazine, or quinoxaline and the like or R1 equals to H, R2 equals to R3 equals to NHCO (CH2) nNR4 or R2 equals to H, R1 equals to R3 equals to NHCO (CH2) nNR4, and m equals to 2 or 3. The invention discloses the preparation method of the quinnazolidone derivative and the purpose of serving as the anticarcinogen at the same time. The quinnazolidone derivative has the advantages of strong inhibitory action on the expression of telomere DNA, c-myc and other proto-oncogenes DNA, obvious inhibitory action on various cancer cell strains, little toxicity on normal cells and wide application space on preparing the anticarcinogen.
Description
technical field The invention belongs to the field of medicine and chemical industry, and relates to a quinazolon derivative, a preparation method thereof, and an application thereof in the preparation of anticancer drugs. Background technique Cancer is a major disease that threatens human health and life safety. According to statistics, there are about 4 million new cancer patients in the world every year. The research and development of anticancer drugs has always been a hot spot for chemists and pharmacologists. Finding anticancer drugs with high efficiency, high selectivity and less toxic side effects is one of the important directions of drug research and development. Designing and synthesizing anticancer drugs with DNA as the target, especially designing and synthesizing small molecule inhibitors for the special high-level structure of proto-oncogene DNA such as telomere DNA and c-myc, which have important physiological significance, is the key to the development of n...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D487/04C07D471/04A61K31/519A61K31/55A61P35/00
Inventor 黄志纾古练权谭嘉恒颜金武候金强
Owner SUN YAT SEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com