Oral sustained-release dry suspension taking azithromycin as main ingredient
A technology of azithromycin and dry suspension, applied in the field of medicine, which can solve the problems of poor drug compliance of patients, inability to effectively mask the bitter taste of drugs, and inaccurate dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
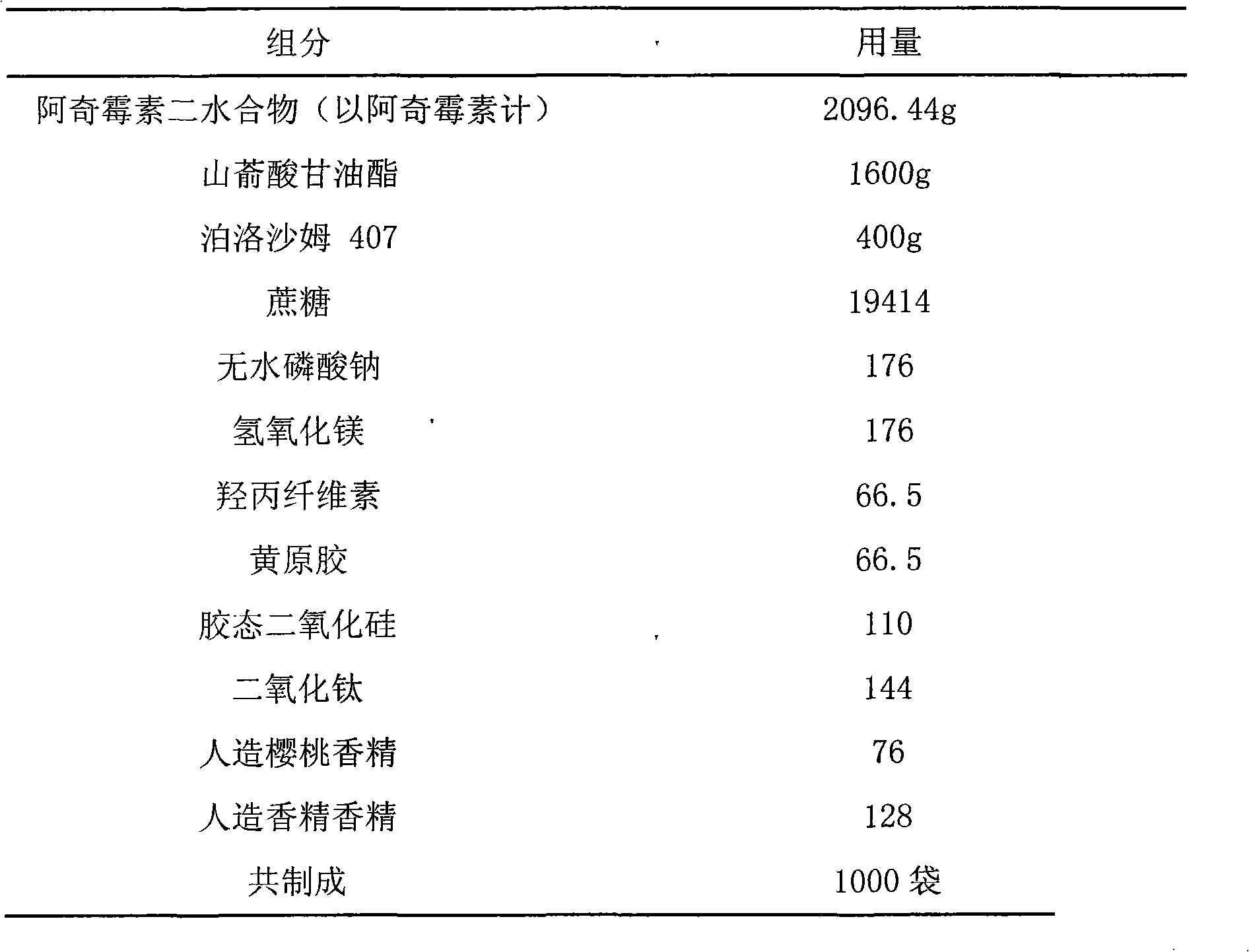
[0026] Embodiment 1 Azithromycin oral sustained-release dry suspension
[0027] prescription:
[0028]
[0029] Preparation:
[0030] a. Take the prescribed amount of azithromycin dihydrate, glyceryl behenate, and poloxamer 407, make the preliminary mixture, pulverize, pass through a 40-mesh sieve, heat to 90°C, melt, and keep at least 80%, preferably 90% of the drug crystalline state.
[0031] b. The drug-loaded molten liquid obtained above is added to a rotary atomizer under the condition of maintaining the temperature, atomized, and cooled to obtain drug-loaded microspheres. The atomizer used is the FX1 100mm rotary atomizer manufactured by Niro A / S.
[0032] c. Fully mix the drug-loaded microspheres obtained in step b with magnesium hydroxide and anhydrous sodium phosphate that have passed through a 40-mesh sieve.
[0033] d. Mix the powder obtained in step c with hydroxypropyl cellulose, xanthan gum, titanium dioxide, artificial cherry flavor, and artificial banana...
Embodiment 2
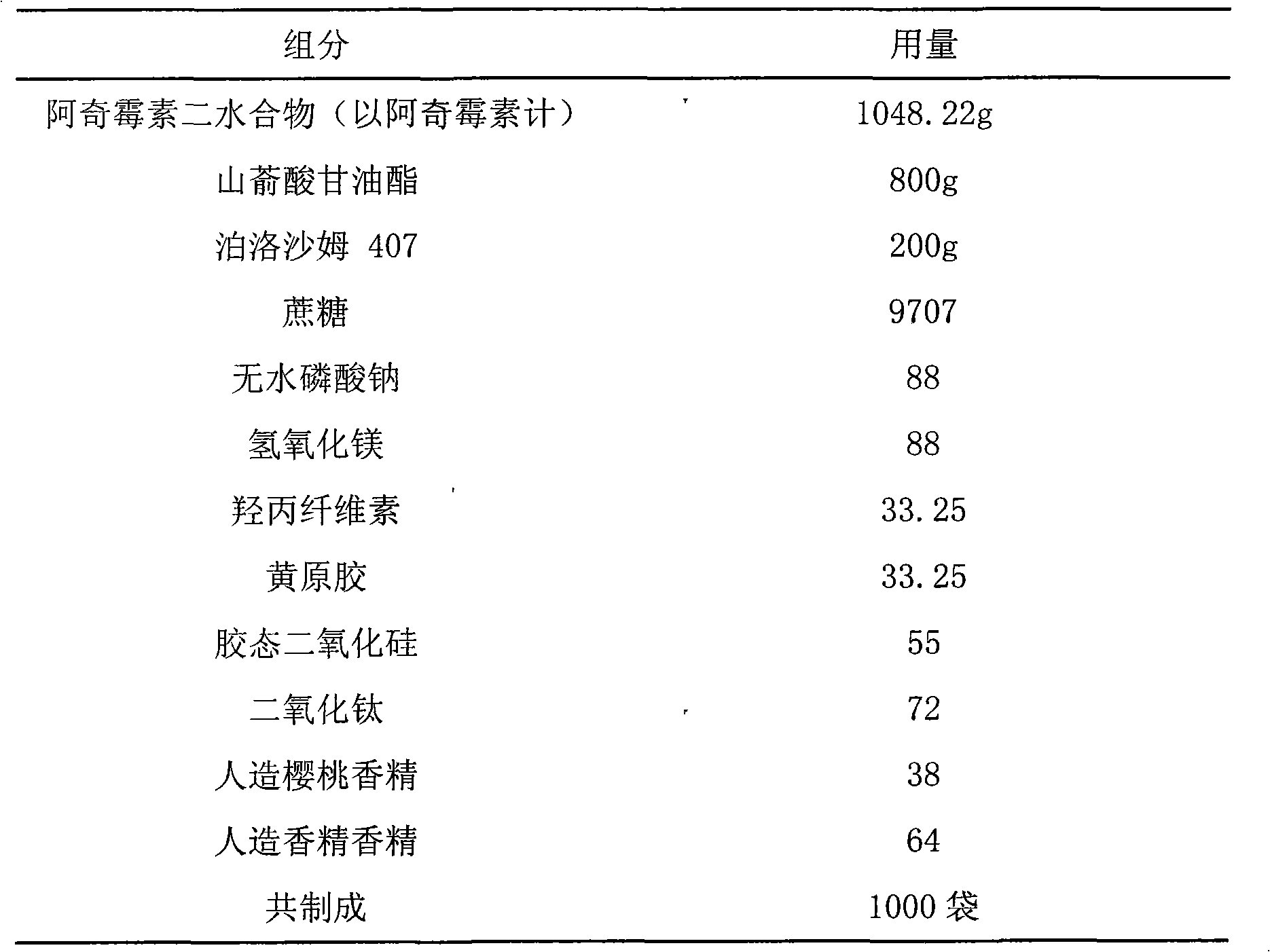
[0035] Embodiment 2 Azithromycin oral sustained-release dry suspension
[0036] prescription:
[0037]
[0038] Preparation:
[0039] a. Take the prescribed amount of azithromycin dihydrate, glyceryl behenate, and poloxamer 407, make the preliminary mixture, pulverize, pass through a 40-mesh sieve, heat to 90°C, melt, and keep at least 80%, preferably 90% of the drug crystalline state.
[0040] b. The drug-loaded molten liquid obtained above is added to a rotary atomizer under the condition of maintaining the temperature, atomized, and cooled to obtain drug-loaded microspheres. The atomizer used is, for example, the FX1100mm rotary atomizer produced by Niro A / S.
[0041] c. Fully mix the drug-loaded microspheres obtained in step b with magnesium hydroxide and anhydrous sodium phosphate that have passed through a 40-mesh sieve.
[0042] d. Mix the powder obtained in step c with hydroxypropyl cellulose, xanthan gum, titanium dioxide, artificial cherry flavor, and artifici...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap