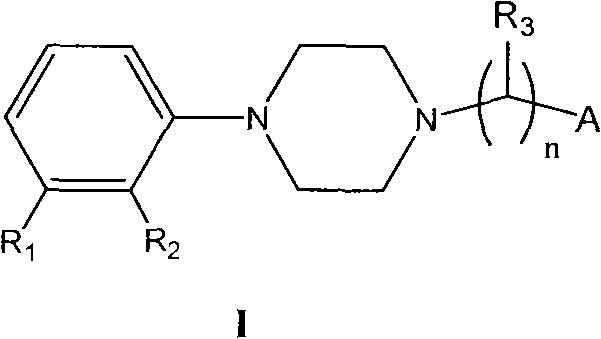
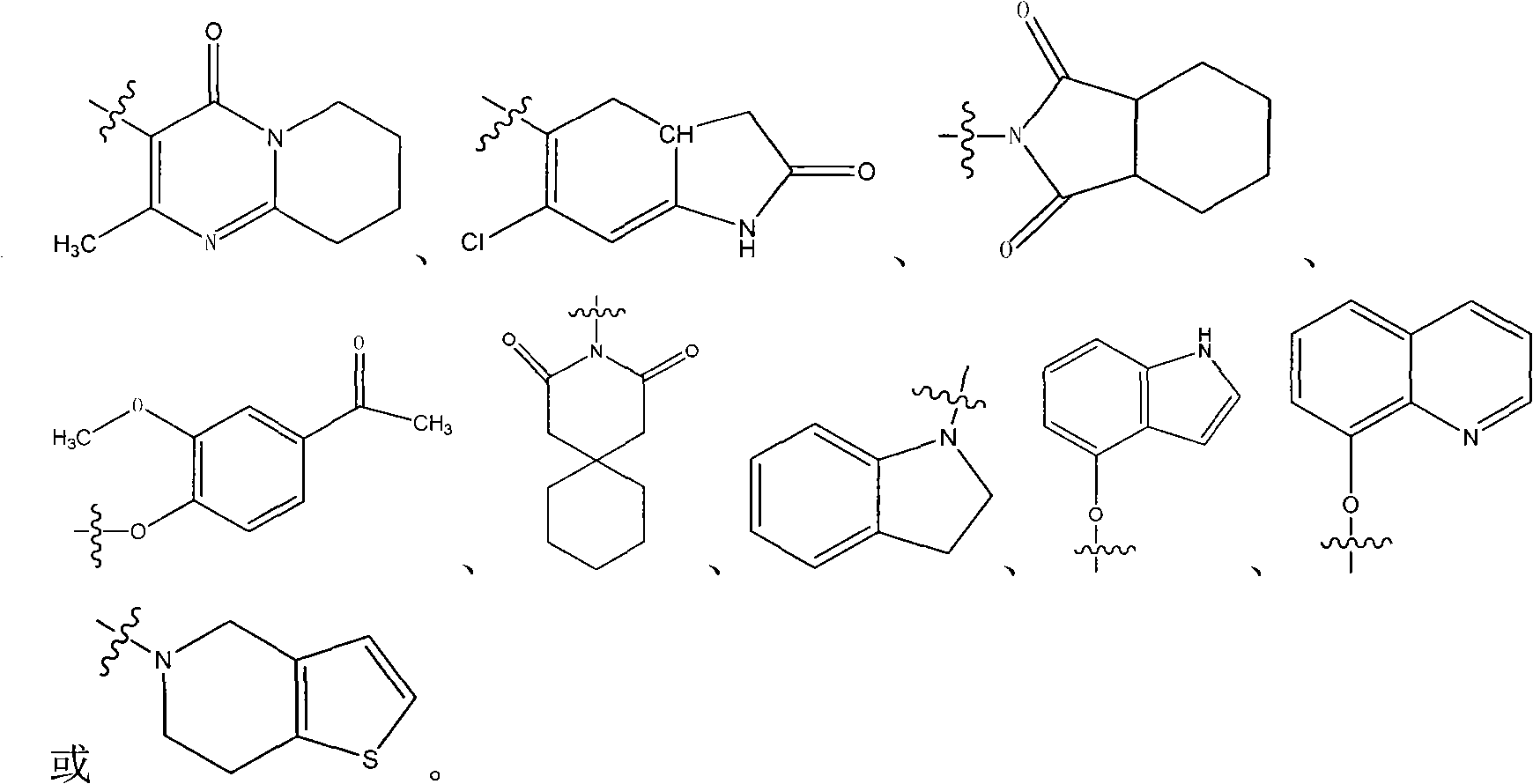
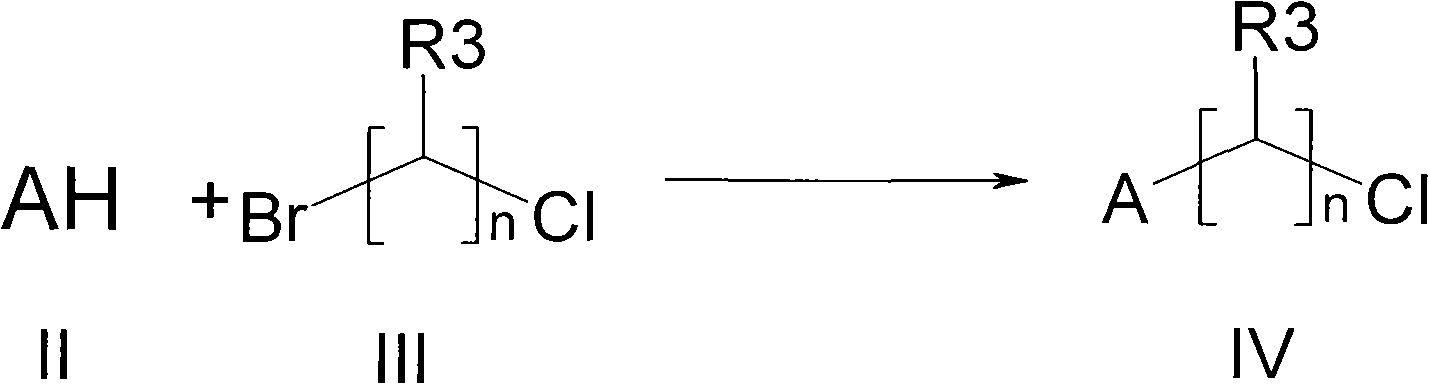N-aryl piperazine derivative having double activity of dopamine D2 and 5-HT2a
A piperazine and alkyl technology, applied in the field of N-arylpiperazine compounds, can solve problems such as adverse reactions and lack of selectivity in action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 3-[2-[4-(2,3-Dichlorophenyl)piperazin-1-yl]ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[ 1,2-a] Preparation of pyrimidin-4-one
[0025] N-(2,3-dichlorophenyl)piperazine hydrochloride (0.6g, 2mmol), 3-(2-chloroethyl)-6,7,8,9-tetrahydro-2-methyl -4H-pyrido[1,2-a]pyrimidin-4-one (0.5g, 2.2mmol), potassium carbonate (0.91g, 6.6mmol) and DMF (10ml) were put into the reaction flask, and the reaction was stirred at 100°C 4 hours. The reaction solution was cooled and poured into water, extracted with ethyl acetate, the organic layer was washed with water and brine in turn, and dried over anhydrous magnesium sulfate. After filtration, the filtrate was concentrated to dryness under reduced pressure, and purified by column chromatography to obtain the title compound: 0.33 g.
[0026] ESI-MS (m / z): 422.36 (M+H),
[0027] 1 H NMR (CDCl 3 , 400M) δ: 1.85-1.97(m, 4H), 2.30(s, 3H), 2.55-2.59(m, 2H), 2.75-2.87(m, 8H), 3.09(t, 4H), 3.93(t, 2H), 6.95(m, 1H), 7.13(m, 2H)
Embodiment 2
[0029] 3-[3-[4-(2,3-dichlorophenyl)piperazin-1-yl]propyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[ 1,2-a] Preparation of pyrimidin-4-one
[0030] The preparation method is the same as in Example 1, except that the reaction raw material is made of 3-(2-chloroethyl)-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2- a] pyrimidin-4-one (0.5g) was changed to 3-(3-chloropropyl)-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a] Pyrimidin-4-one (0.53 g).
[0031] ESI-MS (m / z): 436.2 (M+H)
Embodiment 3
[0033] 3-[4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[ 1,2-a] Preparation of pyrimidin-4-one
[0034] The preparation method is the same as in Example 1, except that the reaction raw material is made of 3-(2-chloroethyl)-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2- a] pyrimidin-4-one (0.5g) was changed to 3-(4-chlorobutyl)-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a] Pyrimidin-4-one (0.56 g).
[0035] ESI-MS (m / z): 450.5 (M+H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com