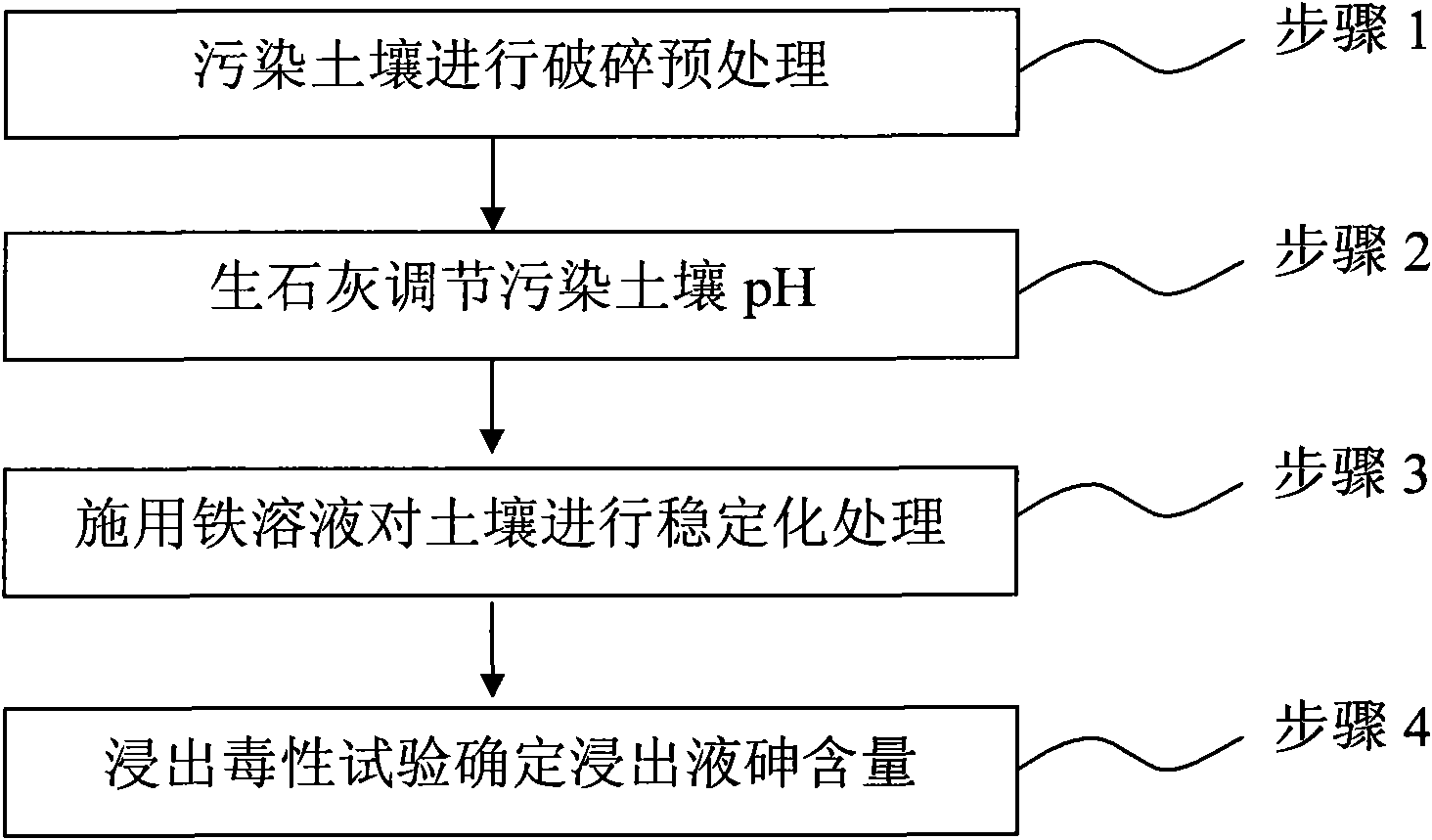
Method for remedying arsenic polluted soil
A technology for arsenic pollution and soil, applied in the restoration of contaminated soil, etc., can solve the problems of inability to remove arsenic efficiently and reliably, and achieve efficient treatment and simple operation and management
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The experimental soil was collected from Haidian District, Beijing, and passed through a 20-mesh nylon sieve after air-drying, impurity removal and grinding. Add NaAsO to arsenic contaminated soil through artificial external sources 2 The form is configured. The artificially configured soil used in this example has an arsenic pollution intensity of 1151 mg / kg and a pH of 8.21. Set the following 7 experimental conditions:
[0049] 1. Fe / As=2, CaO%=0 (that is, no quicklime is added);
[0050] 2. Fe / As=4, CaO%=0;
[0051] 3. Fe / As=6, CaO%=0;
[0052] 4. Fe / As=8, CaO%=0;
[0053] 5. Fe / As=12, CaO%=0;
[0054] 6. Fe / As=16, CaO%=0;
[0055] 7. Fe / As=20, CaO%=0;
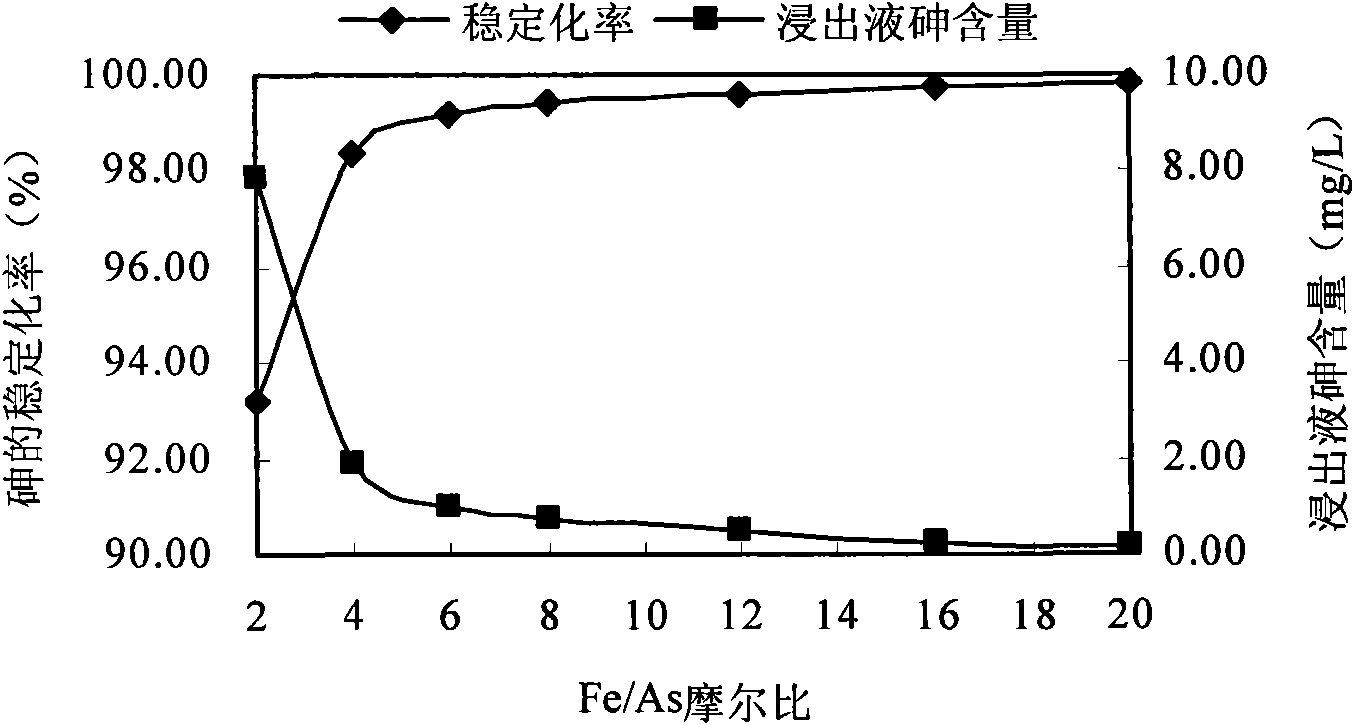
[0056] In this example, the stabilizer FeCl 3 They were added to the contaminated soil at the set molar ratio of Fe / As, and after stabilizing for 1 day, after a toxicity leaching experiment, it was found that as the molar ratio of Fe / As increased, the arsenic content in the leaching solution continued to decrease, and the stab...
example 2
[0058] The soil used in Example 2 was the same as that in Example 1. There are still 7 experimental conditions:
[0059] 1. Fe / As=3, CaO%=0.005%;
[0060] 2. Fe / As=3, CaO%=0.01%;
[0061] 3. Fe / As=3, CaO%=0.05%;
[0062] 4. Fe / As=3, CaO%=0.1%;
[0063] 5. Fe / As=3, CaO%=0.5%;
[0064] 6. Fe / As=3, CaO%=1%;
[0065] 7. Fe / As=3, CaO%=2%;
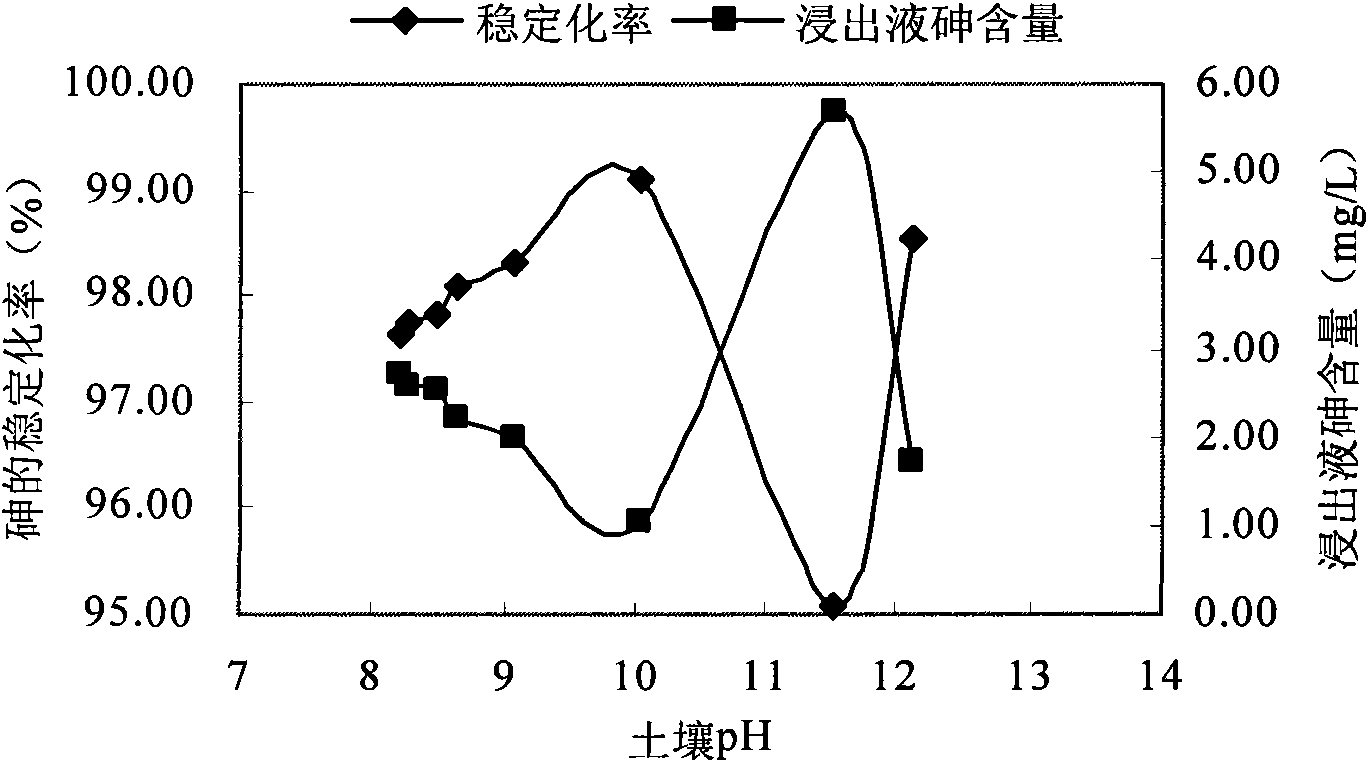
[0066] In this example, before using the stabilizer, first use quicklime to adjust the soil pH. When CaO was added to the contaminated soil at 0.005%, 0.01%, 0.05%, 0.1%, 0.5%, 1%, 2% (mass ratio), the pH of the soil was 8.28, 8.50, 8.63 after being buffered by the soil system. , 9.06, 10.02, 11.52, 12.12. FeCl 3 Add the Fe / As molar ratio of 3 to the contaminated soil sample, keep the solid-to-liquid ratio at about 2:1, continue stirring for 20 minutes, and stabilize for 1 day. The stabilization effect of arsenic varies with soil pH image 3 . It can be seen from the figure that in the range of pH 8.28-10.02, with the increase of pH, the stabilization ...
Embodiment 3
[0068] The three soil samples used in this example are still collected from Haidian District and are artificially configured simulated soil samples. Its characteristics are shown in Table 1. The pH values of soil samples S1, S2 and S3 were 9.41, 10.02, and 9.73 after being buffered by 0.1% quicklime. Then add FeCl with Fe / As=3 3 The solution was stable for 1 day. After the leaching toxicity test, the stabilization rates of arsenic in soil samples S1, S2, and S3 were 97.98%, 99.61%, and 99.22%, respectively, and the arsenic content in the leaching solution was 2.32mg / L and 1.48mg / L and 3.43mg / L are both lower than 5mg / L.
[0069] Table 1 Some characteristics of artificially configured simulated soil samples
[0070]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com