Pyrimidine-ring-contained palladium metal ligand and preparation method thereof
A metal ligand and pyrimidine-based ring technology, applied in the field of organic synthesis, can solve the problems of strong coordination ability and many coordination sites of multidentate ligands, and achieve the effects of stable structure, low requirements for reaction conditions, and convenient storage and transportation.
Inactive Publication Date: 2010-11-17
LUOYANG NORMAL UNIV
View PDF3 Cites 15 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, multi-dentate ligands have many coordination sites and strong coordination ability, and it is easy to generate palladium complexes instead of the required cyclopalladium compounds when directly reacting with palladium sources.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
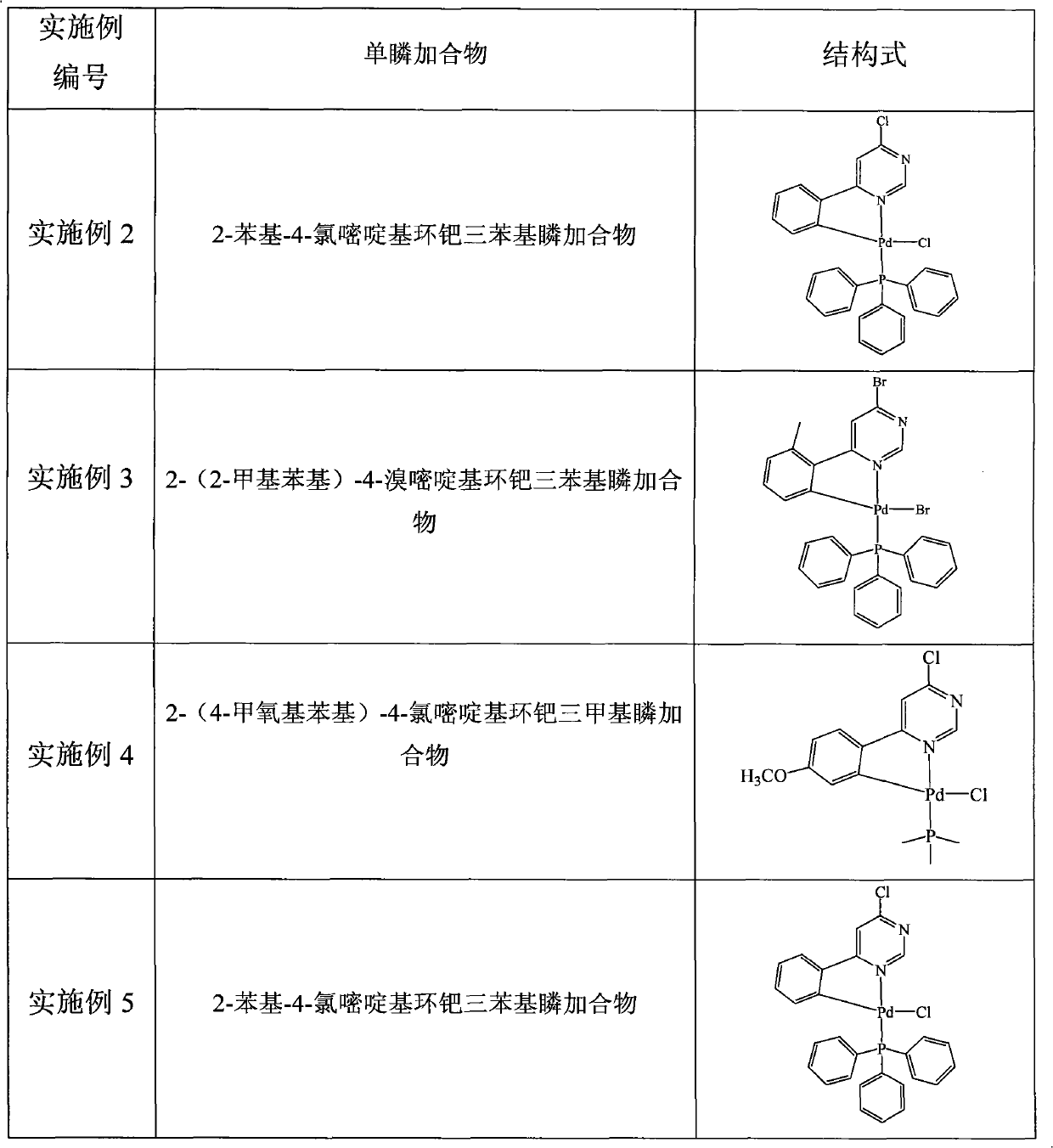
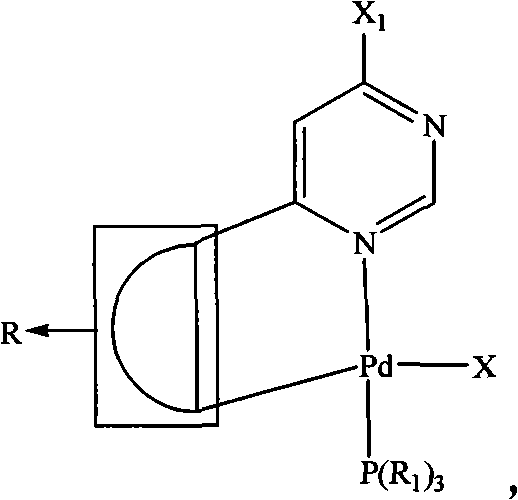
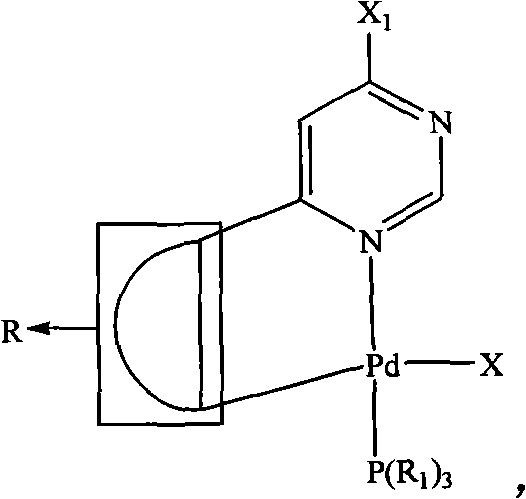
The invention relates to a pyrimidine-ring-contained palladium metal ligand and a preparation method thereof, belonging to the field of organic synthesis. The pyrimidine-ring-contained palladium metal ligand has a general formula which is shown in the specification, wherein the dotted portion is R; R is aryl or ferrocenyl, R1 is phenyl, ring ethyl, ethyl, propyl, or butyl, and Aryl is aryl or heterocyclic group, and X is selected from Cl-, Br- and I-. The preparation method of the pyrimidine-ring-contained palladium metal ligand comprises the following steps of: mixing halogenated pyrimidine ground ring single palladium adduct, Aryl-B(OH)2, alkali and organic solvent; heating and refluxing under the protection of inert gases; adding water after refluence; and then, adding an organic solvent to extract products to obtain a pyrimidine-ring-contained palladium metal ligand. The halogenated pyrimidine ground ring single palladium adduct has a general formula which is shown in the specification, wherein the dotted portion is R; the X1 is Cl-, Br- or I-. The method avoids the direct reaction between a multidentate ligand and a palladium source, avoids adding an additional palladium catalyst in a reaction process, has low requirements for reaction conditions, high yield and stable products and can be used for preparing ring-palladium polynuclear metal compositions.
Description
technical field The invention belongs to the field of organic synthesis, and in particular relates to a class of pyrimidinyl-containing ring palladium metal ligands, and also relates to a preparation method of the compounds. Background technique In recent years, heteropolynuclear metal complexes have outstanding performance in the fields of magnetic exchange of metal ions, electron transfer, bioinorganic chemistry, etc., and have developed into a hot research field, especially in the research of new high-efficiency catalysts and special materials. application. At present, the synthesis methods of heteropolynuclear metal complexes mainly include the following: (1) polymerization of mononuclear complexes; (2) synthesis of complexes as ligands; (3) synthesis by multidentate ligands; (4) Directly synthesized by polyatomic bridging groups; (5) synthesized by substitution reaction of metal ions; (6) spontaneously self-assembled. Among them, the method of "using complexes as liga...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07F19/00C07F15/00C07F17/02B01J31/24
Inventor 徐晨高辉刘辉娄新华李红梅王志强
Owner LUOYANG NORMAL UNIV
Features
- Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com