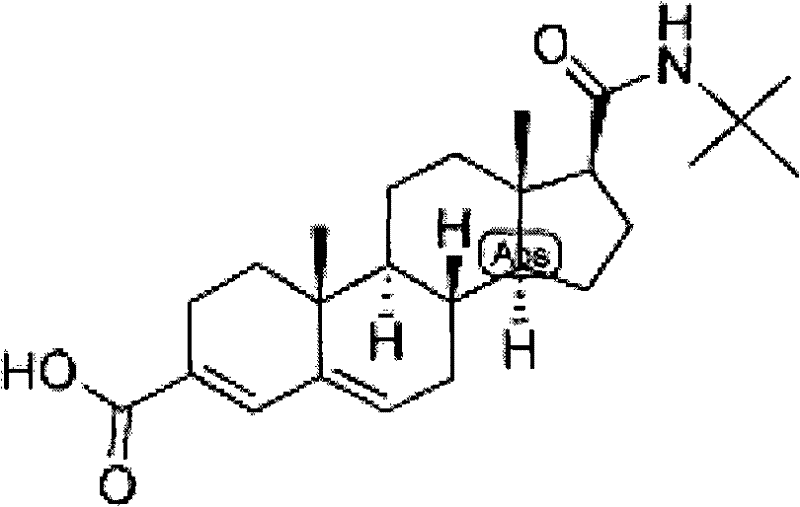
Targeted medicament for curing prostate diseases
A technology for prostate and prostate cancer, applied in drug combination, urinary system diseases, anti-tumor drugs, etc., can solve problems such as unsatisfactory treatment of prostate diseases, toxic side effects, and adverse effects on patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Preparation of Epuletide-PSA Antibody by DCC Method (Method 1)
[0103] EDC (1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride) is a water-soluble carbodiimide used as an activating reagent for carboxyl groups in amide synthesis . The ratio of Epulet to EDC is 1M:1.2-1.8M. The pH range of the activation reaction is 4.0-6.0. After the activation reaction, the PSA antibody (the antibody is a mouse monoclonal antibody) was added, and the ratio of the amount of the PSA antibody added to apretide was 1M:10-100M. The DEAE ion exchange column is used for chromatographic separation, that is, the E-PSA conjugate is suspended in a buffer solution, and the buffer solution uses 100mM Tris, pH8.6, to remove small molecular compounds and obtain a purified conjugate product.
Embodiment 2
[0105] Preparation of Aprelate-PSA Antibody by DCC Method (Method 2)
[0106] EDC (1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride) is a water-soluble carbodiimide used as an activating reagent for carboxyl groups in amide synthesis . The ratio of Epulet to EDC is 1M:1.2-1.8M. The pH range of the activation reaction is 4.0-6.0. After the activation reaction is completed, add PSA antibody, the ratio of the amount of PSA antibody added to eprelate is 1M:10-100M, and a small amount of N-hydroxysuccinimide (NHS) is added at the same time to complete the coupling reaction. The DEAE ion exchange column is used for chromatographic separation, that is, the E-PSA conjugate is suspended in a buffer solution, and the buffer solution uses 100mM Tris, pH8.6, to remove small molecular compounds and obtain a purified conjugate product.
Embodiment 3
[0108] Preparation of Epuletide-PSA Antibody by DCC Method (Method 3)
[0109] Add cyanogen bromide to Apretide for activation (the weight ratio to cyanogen bromide is 1:0.5), and then add adipic hydrazide for treatment, the derivatization rate is 1-3%. After the activation reaction finishes, add PSA antibody and carbodiimide (19.17mg / ml), the ratio of the amount that PSA antibody adds and aprelate is 1M: 10-100M, adds a small amount of N-hydroxyl succinimide ( NHS), together to complete the coupling reaction. The DEAE ion exchange column is used for chromatographic separation, that is, the E-PSA conjugate is suspended in a buffer solution, and the buffer solution uses 100mM Tris, pH8.6, to remove small molecular compounds and obtain a purified conjugate product.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap