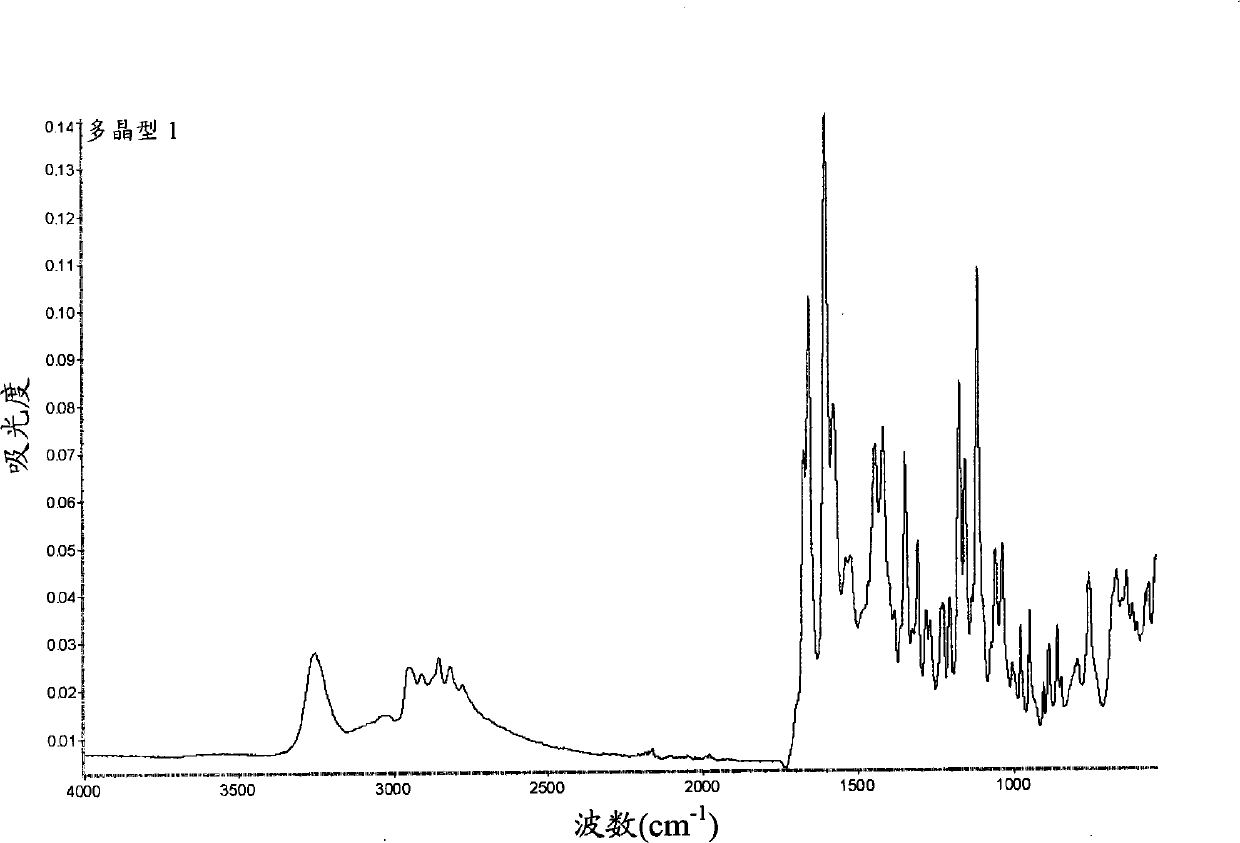
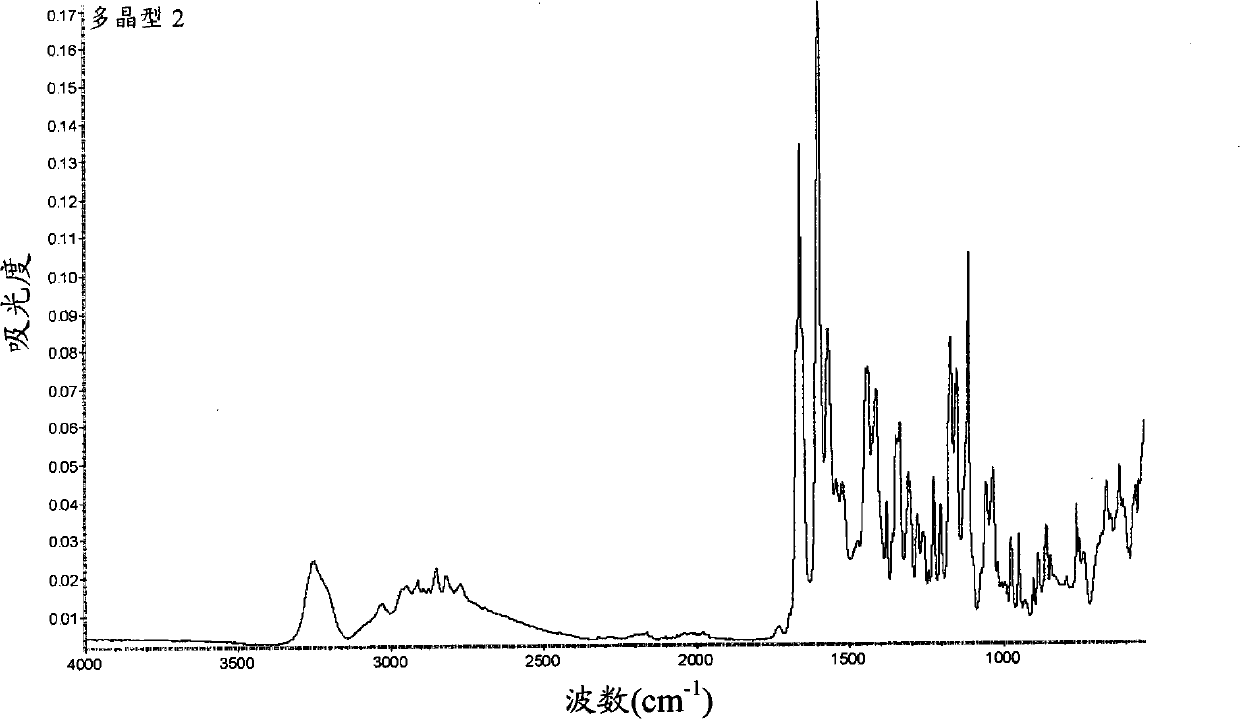
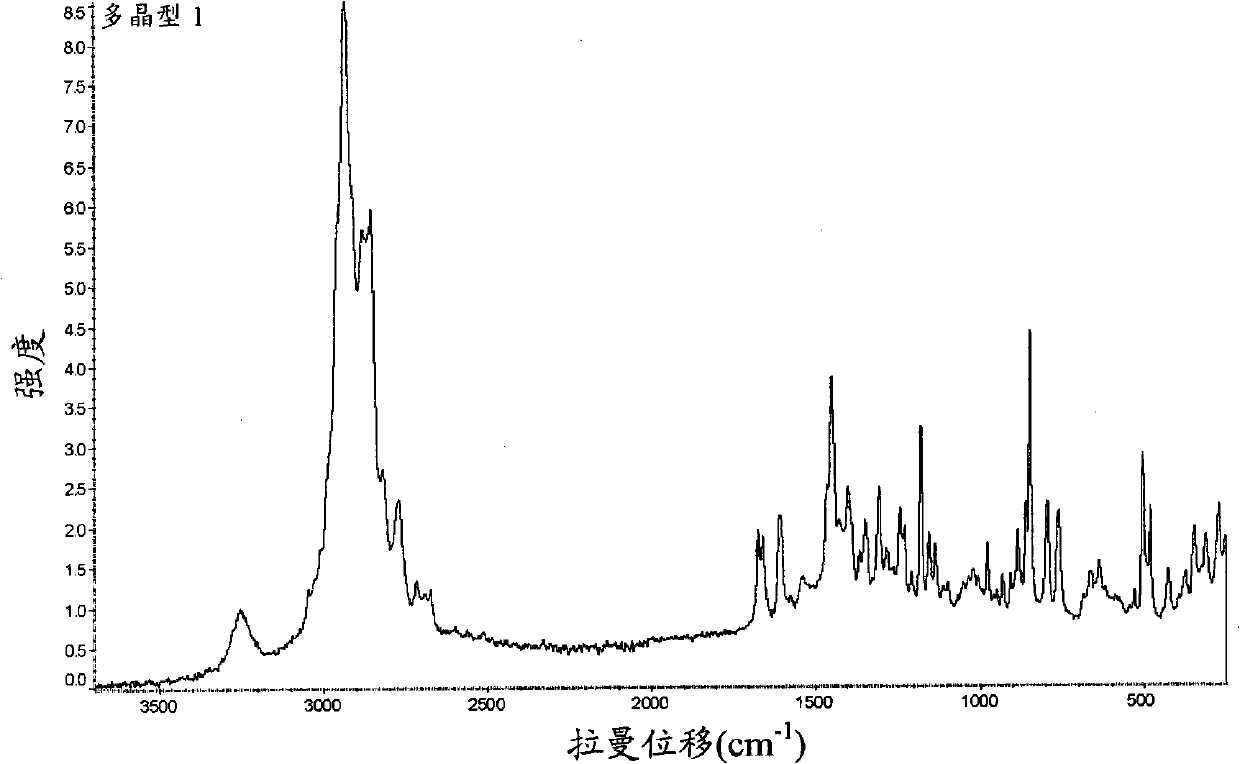
Peptide deformylase inhibitors
A compound, C1-C3 technology, applied in the field of {2--3-[2-hydrazino]-3-oxopropyl}hydroxyformamide compound, can solve the problem of no effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0556] ((2R)-2-(cyclopentylmethyl)-3-{2-[5-fluoro-2-methyl-6-(1-pyrrolidinyl)-4-pyrimidinyl]hydrazino}-3 -Oxopropyl)hydroxyformamide
[0557]
[0558] Part A:
[0559] 5-Fluoro-4-hydrazino-2-methyl-6-(1-pyrrolidinyl)pyrimidine
[0560] 4,6-Dichloro-5-fluoro-2-methylpyrimidine (100 mg, 0.55 mmol) was dissolved in 2 mL of DMSO and stirred at room temperature. Pyrrolidine (50 μL, 0.61 mmol) was added followed by DIPEA (210 μL, 1.21 mmol). The resulting reaction mixture was stirred for 2 hours, and then hydrazine (1.0 mL) was added and the contents heated at 80 °C for 1 hour. The reaction mixture was then cooled to room temperature and purified by RP-HPLC to give 5-fluoro-4-hydrazino-2-methyl-6-(1-pyrrolidinyl)pyrimidine (69 mg, 59%).
[0561] Part B:
[0562] ((2R)-2-(cyclopentylmethyl)-3-{2-[5-fluoro-2-methyl-6-(1-pyrrolidinyl)-4-pyrimidinyl]hydrazino}-3 -Oxopropyl)[(phenylmethyl)oxy]formamide
[0563] 5-Fluoro-4-hydrazino-2-methyl-6-(1-pyrrolidinyl)pyrimidine (69 ...
Embodiment 2
[0569] [(2R)-3-{2-[6-(1-Azetidinyl)-2-ethyl-5-fluoro-4-pyrimidinyl]hydrazino}-2-(cyclopentylmethyl )-3-oxopropyl]hydroxyformamide
[0570]
[0571] Part A:
[0572] 6-azetidinyl-2-ethyl-5-fluoro-4-hydrazinopyrimidine
[0573] 4,6-Dichloro-2-ethyl-5-fluoropyrimidine (195 mg, 1.0 mmol) was dissolved in 3 mL of MeOH and stirred at room temperature. Azetidine (74 μL, 1.1 mmol) was added followed by DIPEA (383 μL, 2.2 mmol). The resulting reaction mixture was stirred at room temperature until the azetidine replaced one chlorine as monitored by LCMS. Then, MeOH was removed in vacuo, and the remaining residue was dissolved in a mixture of 2 mL DMSO and 1 mL hydrazine. The resulting solution was heated at 40 °C for 1 hour until the reaction was deemed complete by LCMS. The crude reaction mixture was purified by RP-HPLC to give 4-(1-azetidinyl)-2-ethyl-5-fluoro-6-hydrazinopyrimidine (136 mg, 64%). LCMS: (M+H) + : 212.1.
[0574] Part B:
[0575] [(2R)-3-{2-[6-(1-Azetidi...
Embodiment 3
[0582] [(2R)-3-{2-[6-(1-Azetidinyl)-5-fluoro-2-(methylthio)-4-pyrimidinyl]hydrazino}-2-(cyclopentyl methyl)-3-oxopropyl]hydroxyformamide
[0583]
[0584] Part A:
[0585] [(2R)-3-{2-[6-(1-Azetidinyl)-5-fluoro-2-(methylthio)-4-pyrimidinyl]hydrazino}-2-(cyclopentyl Methyl)-3-oxopropyl](tetrahydro-2H-pyran-2-yloxy)formamide
[0586] Add [(2R)-3-{2-[6-chloro-5-fluoro-2-(methylthio)-4-pyrimidinyl]hydrazino}-2-(cyclopentylmethyl )-3-oxopropyl](tetrahydro-2H-pyran-2-yloxy)formamide (0.100g, 0.204mmol), azetidine hydrochloride (19.1mg, 0.204mmol), DIPEA (71.2 μL, 0.408 mmol) and DMSO (2 mL). The tube was sealed and heated to 65-70°C with stirring for 3 days. The reaction mixture was then cooled to room temperature and purified by RP-HPLC to give [(2R)-3-{2-[6-(1-azetidinyl)-5-fluoro-2-(methylthio )-4-pyrimidinyl]hydrazino}-2-(cyclopentylmethyl)-3-oxopropyl](tetrahydro-2H-pyran-2-yloxy)formamide (68mg, 65% ). LCMS: (M+H) + : 511.2.
[0587] Part B:
[0588] [(2R)-3-{...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com