Use of l-cysteine ester compounds in inhibiting ndm-1
A technology of cysteine ester and NDM-1, which is applied in the field of pharmacy, can solve the problems of undiscovered L-cysteine ester compounds and achieve the effects of solving drug resistance, eliminating hydrolysis and improving curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
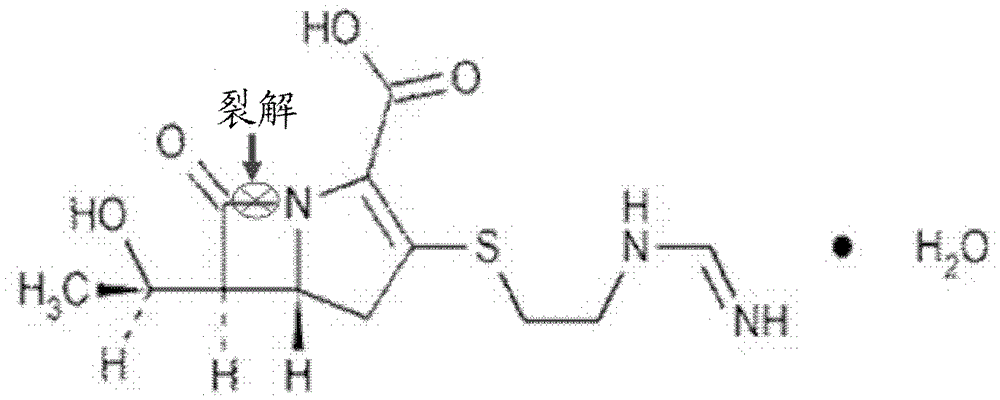
[0037] Step 1. Preparation of NDM-1 substrate stock solution
[0038] Imipenem monohydrate (Imipenemmonohydrate, purchased from Sigma Company) was dissolved in 50 mM HEPES (purchased from BioBasie Company), and prepared into a 10 mM substrate stock solution for future use.
[0039] Step 2. Treatment of Compounds
[0040] Compound in 95%DMSO+5%ddH 2 Dissolve in O, and prepare a solution with a concentration of 100mM, then place the prepared compound solution in a 1.5mlep tube, and store it at 4°C for future use.
[0041] Step 3. Preparation of NDM-1 protein buffer
[0042] Dissolve NDM-1 (provided by the MDC protein purification group of our laboratory, see YuGuo, JingWang et al., Astructural view of the antibiotic degradation enzyme NDM-1 from a superbug. Protein & Cell, 2011, 2(5): 384-394 for the preparation method) in protein buffer (pH=6.8) , prepared into 50nM NDM-1 protein buffer, wherein the protein buffer contains 50mM HEPES, 5μM ZnCl 2 (purchased from BioBasic Compa...
Embodiment 1
[0054] Embodiment 1 N-benzyloxycarbonyl-L-cysteine methyl ester inhibits the determination of NDM-1 activity
[0055]
[0056] The N-benzyloxycarbonyl-L-cysteine methyl ester used was purchased from Bailingwei with a purity of 98%.
[0057] N-benzyloxycarbonyl-L-cysteine methyl ester (4 mg) was dissolved in 95% DMSO double distilled water (148.5 μL) to prepare a solution with a concentration of 100 mM, and then the solution was placed in a 1.5 mlep tube, Store at 4°C.
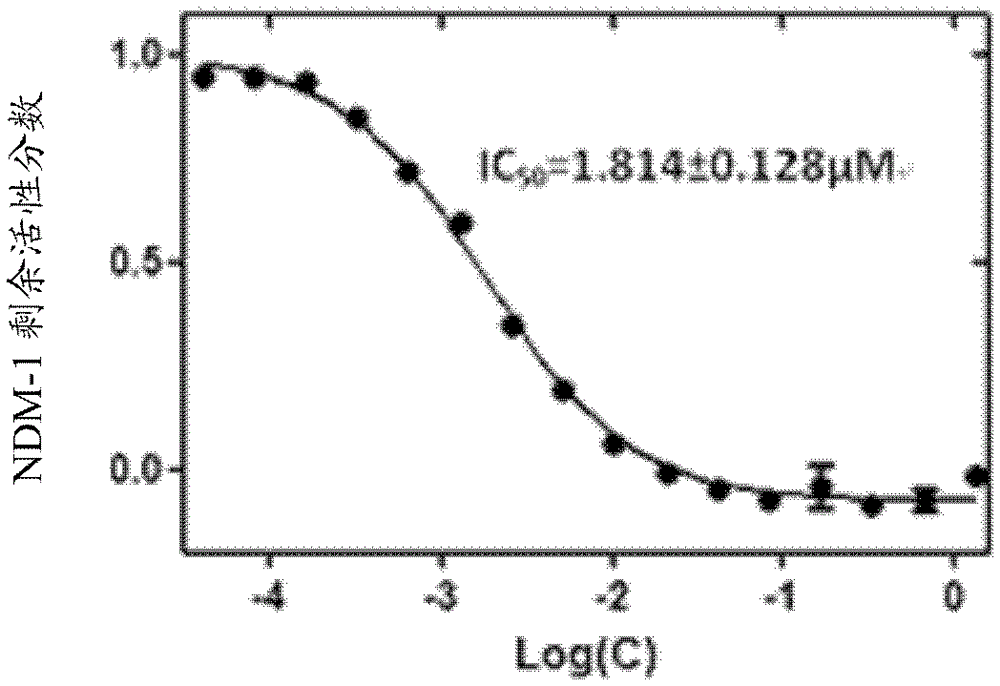
[0058] Then according to the above activity test method step 5 (preliminary screening of compound) and step 6 (IC of compound) 50 Determination of the value) described in the operation. Then take the concentration logarithm of N-benzyloxycarbonyl-L-cysteine methyl ester as the abscissa, and the remaining active fraction of NDM-1 as the ordinate to draw a curve, see image 3 . Finally, according to the curve, using GraphPad Prismversion5.0 software to calculate, the obtained IC 50 The value is 1....
Embodiment 2
[0059] Example 2 Determination of NDM-1 activity inhibited by N-acetyl-L-cysteine methyl ester
[0060]
[0061] The N-acetyl-L-cysteine methyl ester used was purchased from Bailingwei with a purity of 99%.
[0062] N-acetyl-L-cysteine methyl ester (4 mg) was dissolved in 95% DMSO double-distilled water (225.8 μL) to prepare a solution with a concentration of 100 mM, and then the solution was placed in a 1.5 mlep tube, and the Store at 4°C.
[0063] Proceed as described in steps 5 and 6 of the activity test method above. Then take the logarithm of the concentration of N-acetyl-L-cysteine methyl ester as the abscissa, and the remaining active fraction of NDM-1 as the ordinate to draw a curve, see Figure 4 . Finally, according to the curve, the GraphPad Prismversion5.0 software is used to calculate the obtained IC 50 The value is 33.94±2.8 μM.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com