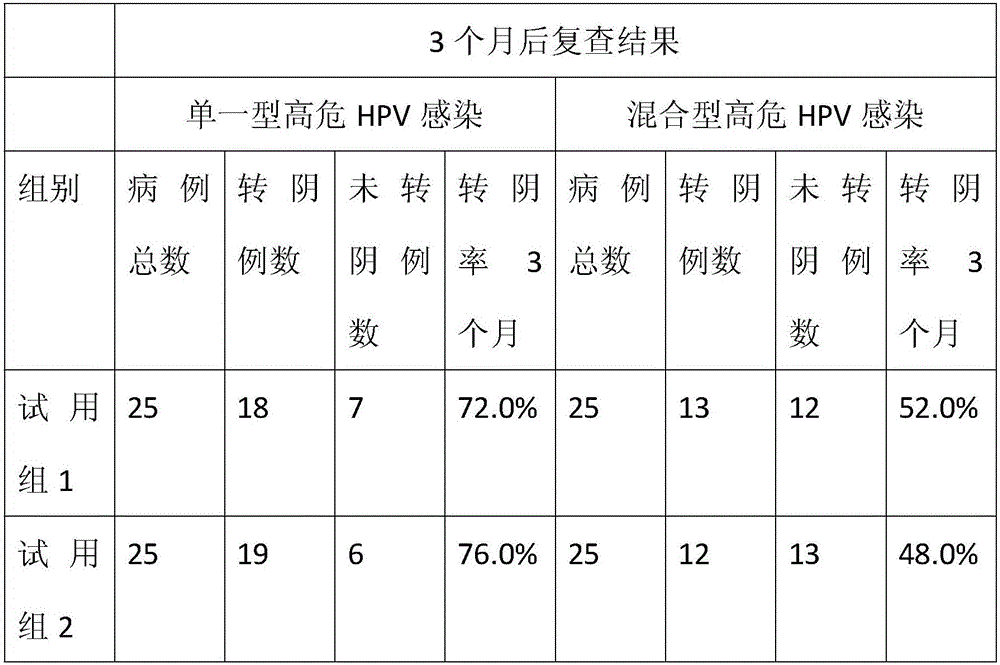
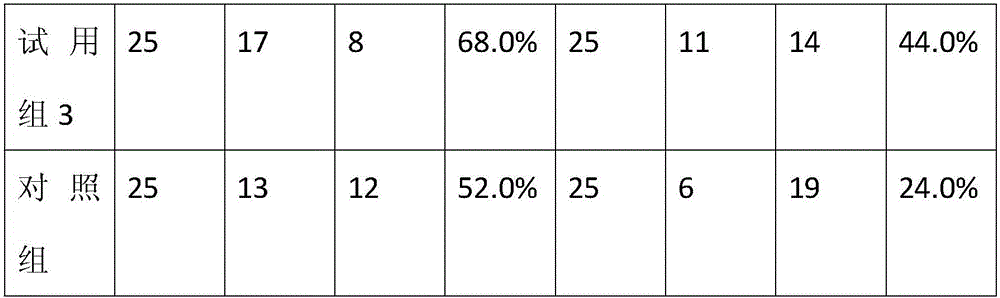
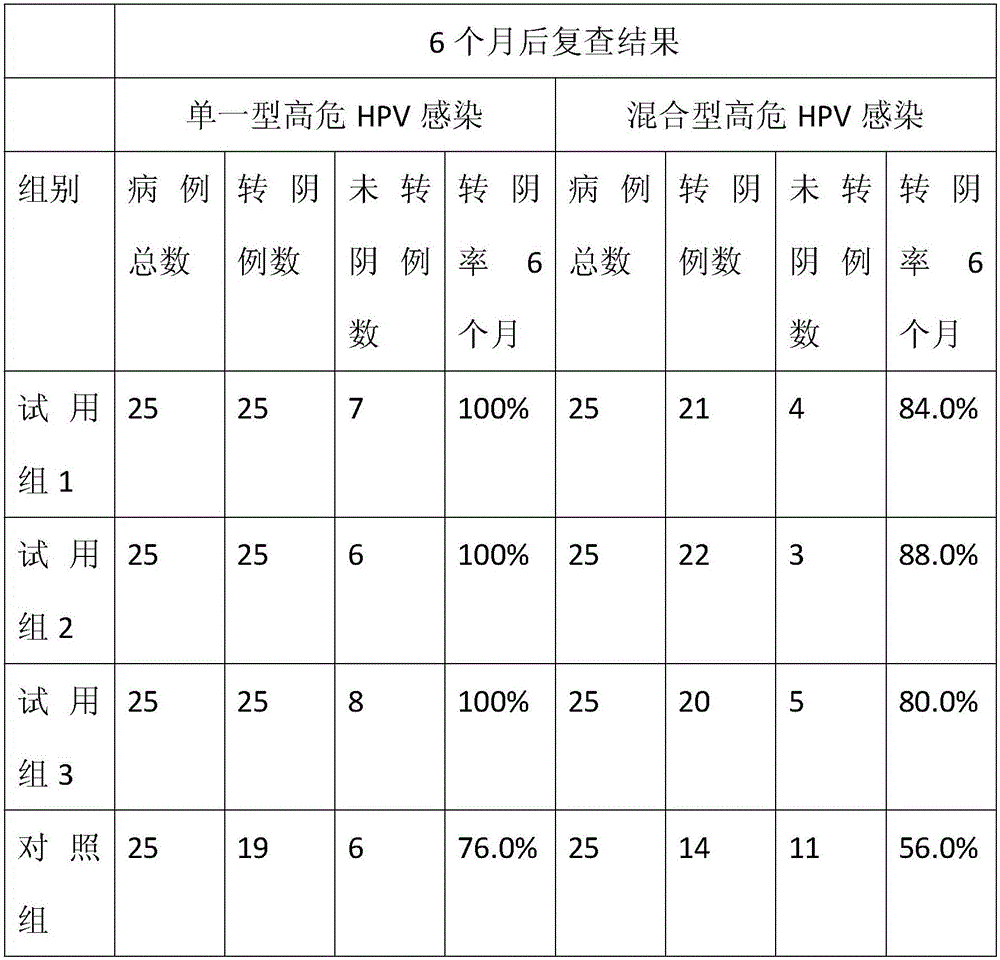
Lactalbumin-oleic acid compound and carrageenan composite medicine as well as preparation method and application thereof
The technology of oleic acid complex and lactalbumin is applied in the field of combined medicine of lactalbumin-oleic acid complex and carrageenan and its preparation, can solve the problems of insufficient effect and the like, achieves high negative conversion rate, convenient use and good curative effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Combination Drugs
[0029] The combined medicine consists of 0.5 parts by weight of lactalbumin-oleic acid complex, 1 part by weight of carrageenan, 2 parts by weight of carbomer, 5 parts by weight of glycerin, 0.15 parts by weight of ethylparaben, 1 part by weight of ethyl paraben, Triethanolamine, the water composition of 90.35 parts by weight.
[0030] Preparation:
[0031] 1): Weigh each raw material according to the ratio, stir 2 parts of carbomer in 20 parts of water at 80°C until fully swollen, then add 1 part of carrageenan, stir until completely dissolved, and filter and sterilize through a 0.22 μm membrane while it is hot , to obtain No. 1 solution;
[0032] 2): Dissolve 0.15 parts of ethylparaben in 5 parts of water, filter and sterilize through a 0.22 μm membrane to obtain No. 2 solution;
[0033] 3): Add No. 2 solution to No. 1 solution under agitation and mix evenly, add 0.5 parts of triethanolamine, adjust the pH to 5.6, and obtain No. 3 sol...
Embodiment 2
[0037] Embodiment 2: combination medicine
[0038] The combined medicine consists of 0.01 weight part of lactalbumin-oleic acid complex, 0.5 weight part of carrageenan, 1 weight part of carbomer, 1 weight part of glycerin, 0.1 weight part of ethylparaben, 1 weight part The triethanolamine, the water composition of 96.39 parts by weight.
[0039] Preparation:
[0040] 1): Weigh each raw material according to the ratio, stir 1 part of carbomer in 20 parts of water at 85°C until fully swollen, then add 0.5 part of carrageenan, stir until completely dissolved, and filter and sterilize through a 0.22 μm membrane while it is hot , to obtain No. 1 solution;
[0041] 2): Dissolve 0.1 part of ethylparaben in 5 parts of water, filter and sterilize through a 0.22 μm membrane to obtain No. 2 solution;
[0042] 3): Add No. 2 solution to No. 1 solution under agitation and mix evenly, add 0.5 parts of triethanolamine, adjust the pH to 5.6, and obtain No. 3 solution;
[0043] 4): Dissolve...
Embodiment 3
[0046] Embodiment 3: combination medicine
[0047] The combination medicine consists of 1 weight part of lactalbumin-oleic acid complex, 1.5 weight parts of carrageenan, 3 weight parts of carbomer, 5 weight parts of glycerin, 0.2 weight parts of ethylparaben, 2 weight parts The triethanolamine, the water composition of 87.3 parts by weight.
[0048] Preparation:
[0049] 1): Weigh each raw material according to the ratio, stir 3 parts of carbomer in 20 parts of water at 85°C until fully swollen, then add 1.5 parts of carrageenan, stir until completely dissolved, and filter and sterilize through a 0.22 μm membrane while it is hot , to obtain No. 1 solution;
[0050] 2): Dissolve 0.2 parts of ethylparaben in 5 parts of water, filter and sterilize through a 0.22 μm membrane to obtain No. 2 solution;
[0051] 3): Add No. 2 solution to No. 1 solution under agitation and mix evenly, add 1 part of triethanolamine, adjust the pH to 5.6, and obtain No. 3 solution;
[0052] 4): Diss...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com