Hypocrellin derivative as well as preparation method and application thereof
A technology of oleocanthal and derivatives, which is applied in the directions of pharmaceutical formulations, drug combinations, photodynamic therapy, etc., can solve the problems of low yield of synthetic derivatives, reduced quantum yield, increased drug cost, etc., and achieves high conversion rate and high efficiency. Productivity, ease of operation, the effect of solving the problem of scar-free treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Preparation of Compound H498
[0066] Bamboo Rhododendron A (HA) 31mg (5.7×10 -5 mol), solid potassium hydroxide 130mg (2.3×10 -3 mole) was added to 20 mL of dimethylformamide, mixed thoroughly, heated to 130°C under the protection of argon, and reacted with electromagnetic stirring for 3.5 hours. was extracted with chloroform for three times, combined, and drained to obtain a black powder, which was recrystallized from dichloromethane / petroleum ether to obtain a brown powder, 25.3 mg of oleocanthin 498 (H498), with a yield of 89.5%.
[0067] The structural detection data of this product are shown below:
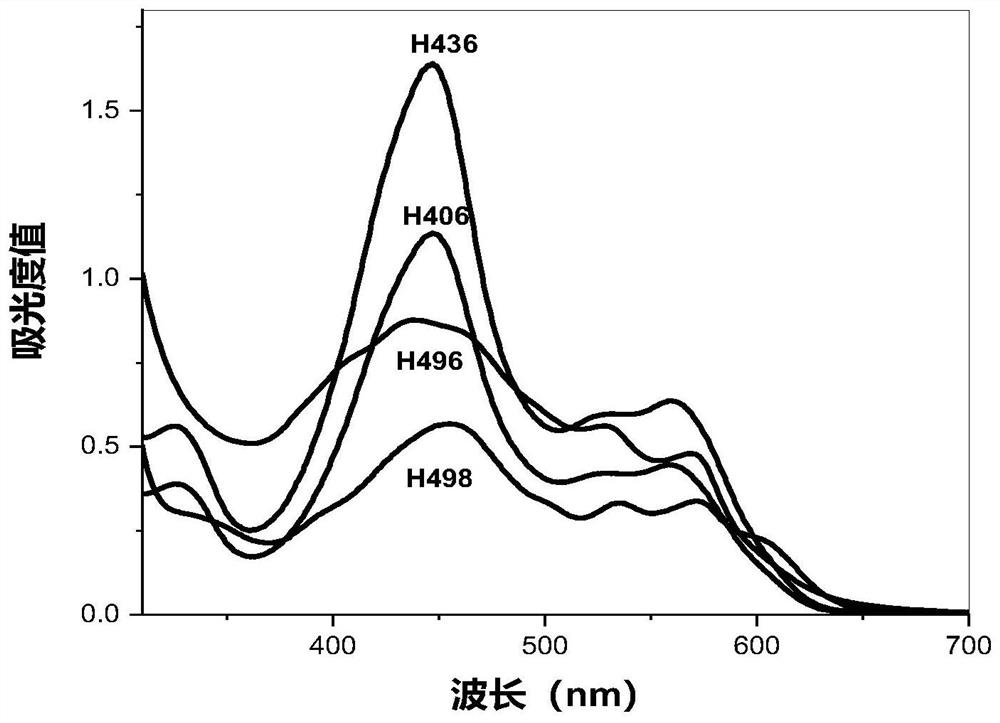
[0068] UV spectrum λ max (CHCl 3 ): 455.5, 530.5, 571.5nm;
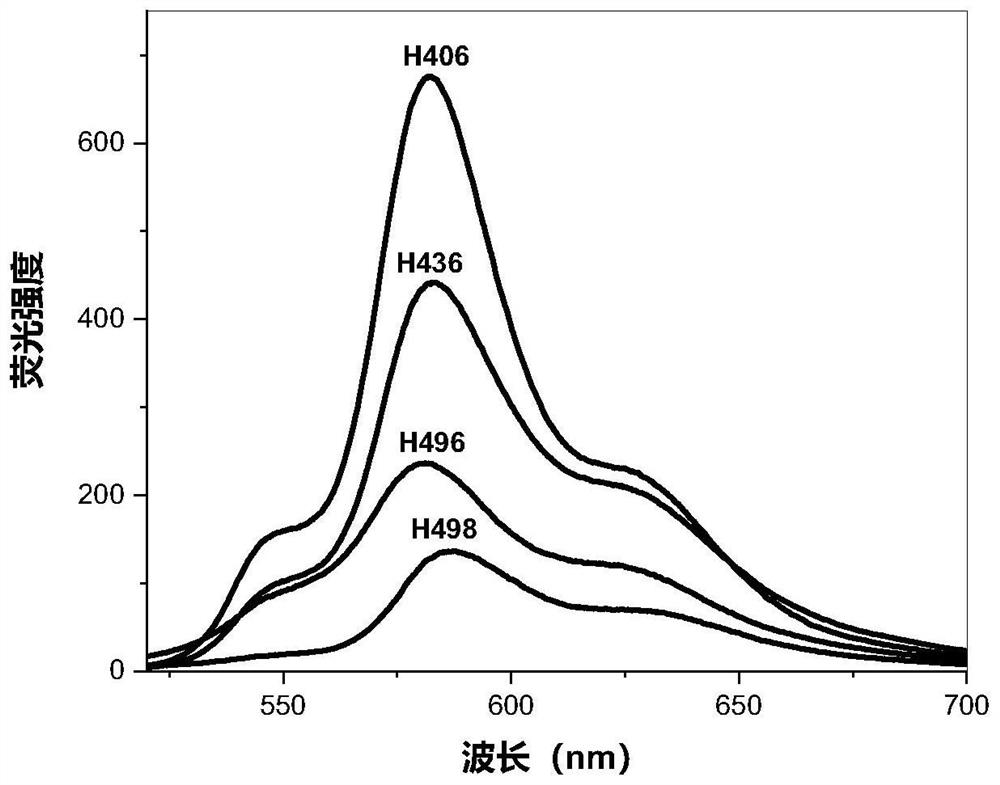
[0069] Fluorescence Spectrumλ max (CHCl 3 ): 587.6nm, 624nm (shoulder peak);
[0070] Infrared spectrumν max : 3433cm -1 ,2541cm -1 ,1727cm -1 ,1623cm -1 ,1395cm -1 ,;
[0071] NMR: δ(1H): 16.69, 16.61(2H), 6.81(s), 6.67(2H), 4.77, 4.62, 4.30(6H), 4.17(s)-3.15(9H), 1.12(3H);
[007...
Embodiment 2
[0074] Example 2: Preparation of compound H496
[0075] Bamboo Rhododendron A (HA) 44mg (8.05×10 -5 mol), with solid potassium hydroxide 0.9g (1.6×10 -2 mol) was added to 25 mL of dimethylformamide, fully mixed, heated to 137° C. under nitrogen protection, and reacted with electromagnetic stirring for 5 hours. After 24 hours, neutralize with an appropriate amount of dilute aqueous hydrochloric acid, extract with chloroform three times, combine, wash with water until neutral, and drain to obtain brown powder. Dichloromethane / petroleum ether was recrystallized to obtain 32.9 mg of brown-red powder, which was oleocorthin 496 (H496), and the yield was 82.4%.
[0076] The structural detection data of the product are shown below:
[0077] UV spectrum λ max (CHCl 3 ): 438.5nm, 528.5nm, 569.5nm;
[0078] Fluorescence Spectrumλ max (CHCl 3 ): 581.2nm, 624nm (shoulder peak);
[0079] Infrared spectrumν max : 3431cm -1 ,2942cm -1 ,1730cm -1 ,1609cm -1 ,1458cm - ;
[0080]...
Embodiment 3
[0087] Example 3: Preparation of Compound H436
[0088] Bamboo Rhododendron B (HB) 41.0mg (7.76×10 -5 mol), solid potassium carbonate 1.1g (1.96×10 -2 mole) was added to 40 mL of dimethylformamide solution, heated to 135°C under nitrogen protection, and reacted with electromagnetic stirring for 4.5 hours. Hour, extracted with dichloromethane 3 times and combined, washed with water until neutral, and drained to obtain a brown-red solid, which was recrystallized from dichloromethane / petroleum ether to obtain 24.1 mg of a brown-red solid, which was oleocanthin 436 (H436), and the yield was 24.1 mg. was 71.1%.
[0089] The structural detection data of this product are shown below:
[0090] UV spectrum λ max (CHCl 3 ): 326.0nm, 450.5nm, 560.5nm;
[0091] Fluorescence Spectrumλ max (CHCl 3 ): 583.6nm;
[0092] Infrared spectrumν max : 2917cm -1 , 1720cm-1 , 1622cm -1 ,1458cm -1 ,1223cm -1 ;
[0093] NMR: δ(1H): 16.87, 16.63(2H), 7.79, 7.58, 7.29(3H) 6.99, 6.44(2H), 4....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com