Modified crystallization purifying and precipitating method of piperacillin acid
A technology of piperacillin acid and piperacillin is applied in the field of improved crystallization, purification and precipitation of piperacillin acid, which can solve the problems of no fluidity, poor filterability, low production performance and the like, and achieves low residue, high content, The effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0007] Example 1: The ES2321153 seedling transplanting method described in the background art was adopted.
[0008] Add 800ml purified water to a flask and 400g crude piperacillin, HPLC is 98%, add 10% sodium bicarbonate solution dropwise to adjust PH=6-6.5 and stir to dissolve completely, add 400ml ethyl acetate at 20-25℃, Stir for 15-20min, separate layers, add 1600ml ethyl acetate to the water phase, then adjust pH=2.0 with 2N hydrochloric acid, stir for 2.0h at 20-25℃, filter, and bake at 40℃ vacuum degree> 0.9MPa Material, 360g piperacillin acid was obtained. Appearance is off-white, needle-like powder crystal HPLC99%, content is 96.8%, total impurities 2.0%, ethyl acetate residue is 7200PPm.
Embodiment 2
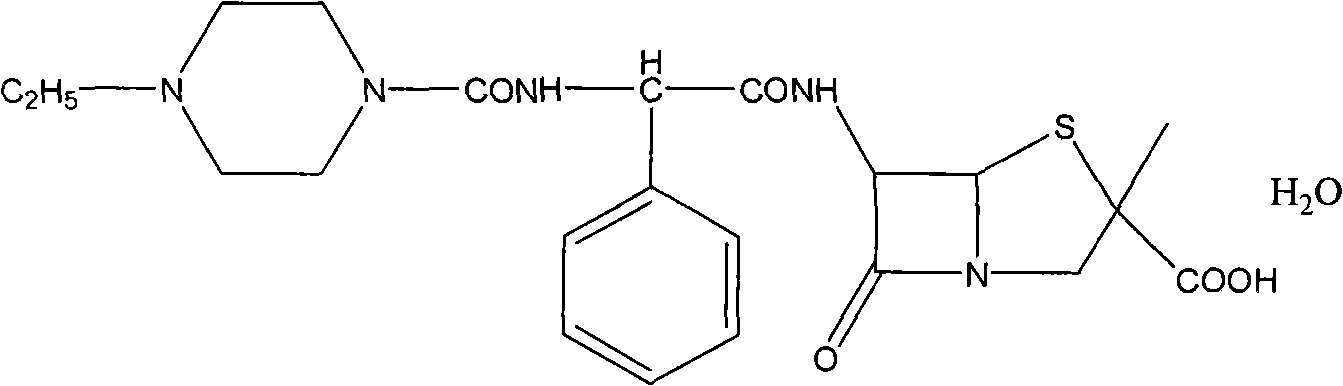
[0010] Add 20g crude piperacillin to 100ml water, cool to 10-20℃, add 8% NaHCO dropwise 3 Aqueous solution, stir to clear, filter, then add filtered ethyl acetate and acetone mixture (mixing ratio of ethyl acetate in the mixture is 30% by volume) about 200ml, then use refined hydrochloric acid to adjust the pH = 1.5-2.0 , Then cooled to 0-5°C, crystallized for 2.0-3.0h, filtered, vacuum-baked at 50-55°C to obtain 18.6g of white granular crystalline piperacillin acid monohydrate. Its chemical structure is shown below, HPLC≥99.6 %, the content is: 99.9%, single impurity <0.1%, residual ethyl acetate and acetone ≤3000ppm.
[0011]
Embodiment 3
[0013] Add 20g of crude piperacillin to 200ml of acetone, heat to reflux to dissolve, filter while hot, cool the filtrate to 10-20°C, add 150ml of ethyl acetate dropwise (equivalent to the volume of ethyl acetate and acetone mixture The percentage is 42.8%), then cool to 0-5°C, crystallize for 2.0-3.0h, filter, and then wash with a small amount of 10ml of acetone at 0-5°C. Vacuum drying at 40-50°C to obtain 18.1 g of white granular crystals of piperacillin acid monohydrate. The chemical structure is as in Example 1, HPLC≥99.6%, content: 100.2%, single impurity <0.1%, residual Ethyl acetate and acetone≤3000ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com