Soluble conductive polymer and method for preparing same
一种导电聚合物、溶液的技术,应用在可溶性导电聚合物及其制备领域,能够解决常规导电聚合物难等问题,达到降低制备成本、制备成本低的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1: Preparation of conductive polymer polyaniline and use it to form conductive film
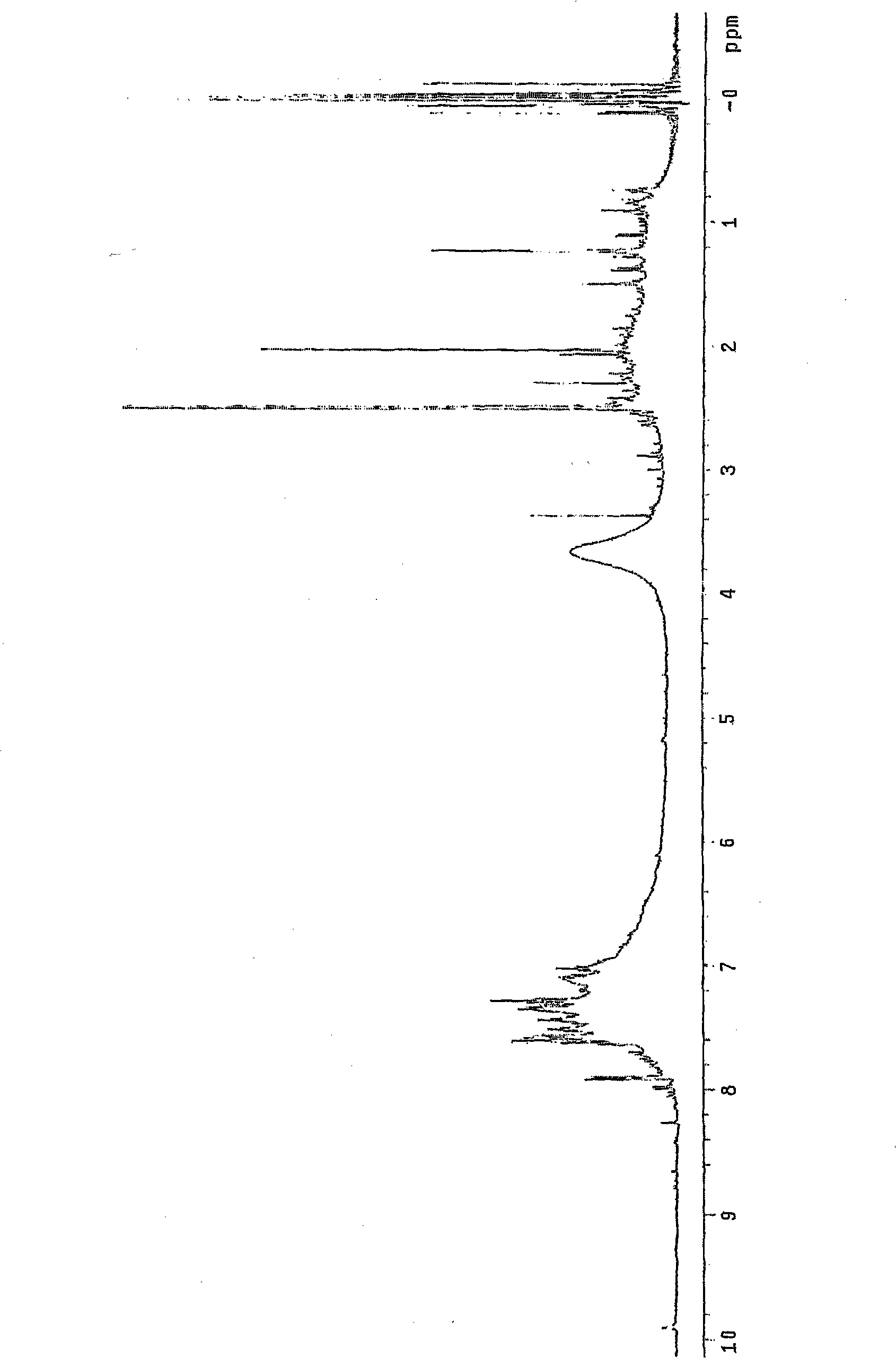
[0074] At room temperature, 0.2 mol of aniline monomer was placed in an Erlenmeyer flask containing 600 ml of methyl ethyl ketone, and stirred using a magnetic stirrer. In this solution, add 0.1 mole of sulfuric acid as doping agent, stir for 30 minutes, add 0.25 mole of ammonium persulfate ((NH 4 ) 2 S 2 o 8 ), reacted for 48 hours or longer (when the viscosity of the solution increases and thus precipitates are generated, 5% by weight or more of another solvent other than methyl ethyl ketone is added based on the total weight of the solution, thereby enabling stirring to be performed) . The reaction solution was filtered to generate a conductive polymer solution.
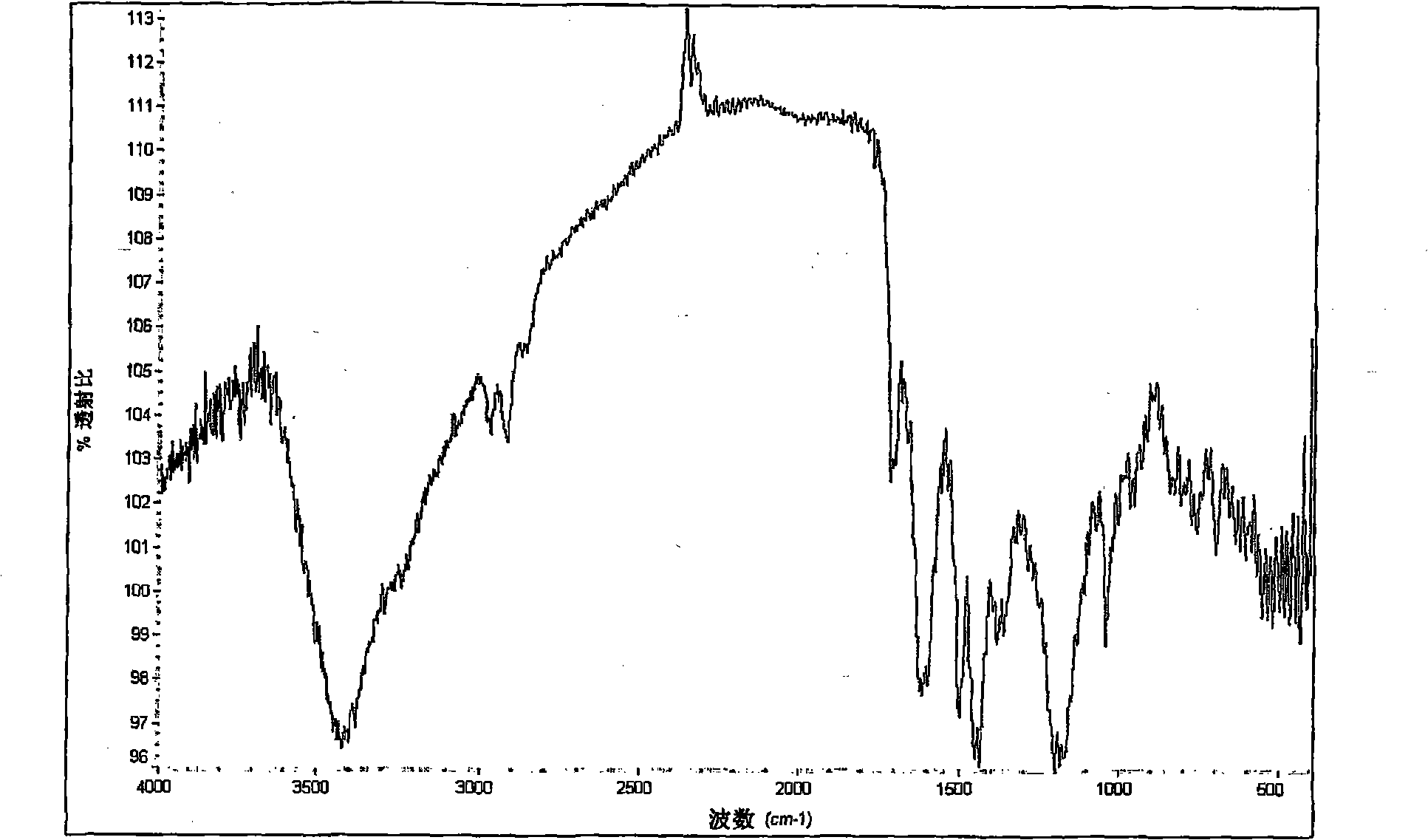
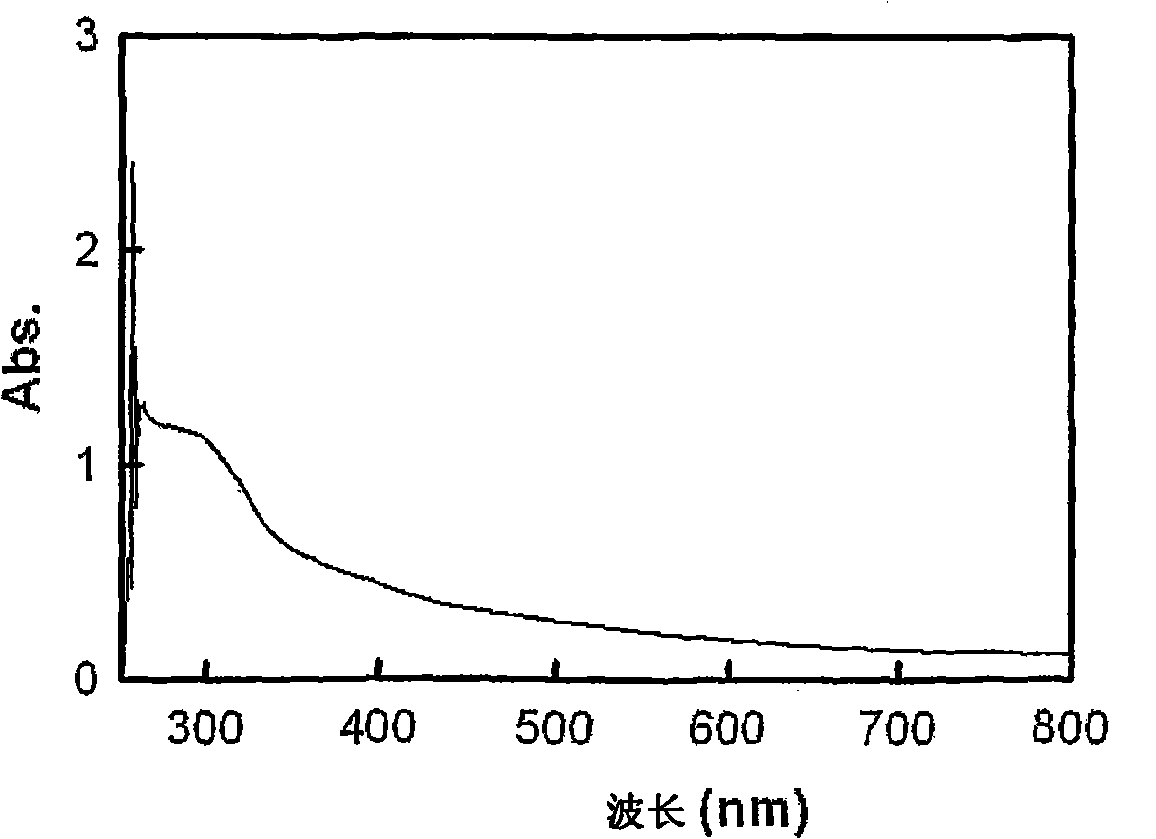
[0075] A thin film was fabricated using this conductive polymer solution, and its conductivity, transmittance, and sheet resistance were measured. The result is a film with 10 -4 S / cm conductivity, and a ...
Embodiment 2
[0077] Embodiment 2: Preparation of conductive polymer polyaniline and use it to form conductive film
[0078] At room temperature, 0.2 mol of aniline monomer was placed in an Erlenmeyer flask containing 600 ml of methyl ethyl ketone, and stirred using a magnetic stirrer. In this solution, add 10 milliliters of water and 0.1 mole of sulfuric acid as doping agent, further add 10 milliliters of N-methylpyrrolidone (NMP) as doping aid, stir for 30 minutes, add 0.25 mole of ammonium persulfate (NH 4 ) 2 S 2 o 8 ), then react for 24 hours or more (when the viscosity of the solution increases and thus precipitates are generated, adding 5% by weight or more of another solvent other than methyl ethyl ketone based on the total weight of the solution, thereby enabling stirring ). The reaction solution was filtered to generate a conductive polymer solution.
[0079] Thin films were fabricated using this conductive polymer solution, and their conductivity, transmittance, and sheet re...
Embodiment 3
[0080] Embodiment 3: Preparation of conductive polymer polyaniline and use it to form conductive film
[0081] Conductive polymer solution is prepared in the same manner as in Example 2, except that 10 milliliters is selected from dimethylsulfoxide (DMSO), dimethylformamide (DMF), methanol, ethanol, propanol, butanol , isopropanol, tetrahydrofuran (THF), ethyl acetate, and butyl acetate in place of 10 ml of NMP. Using the conductive polymer solution thus prepared to produce a film, the conductivity, transmittance and sheet resistance of the film were almost the same as those when 10 ml of NMP was used as a doping aid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight-average Molecular Weight | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com