Chiral binaphthyl chromatogram immobile phase, and preparation method and application thereof
A technology of chromatographic stationary phase and binaphthyl, which is applied in the field of chromatographic stationary phase of chemical technology, can solve problems such as cumbersome bonding methods, and achieve the effects of simple preparation method, mild reaction conditions, and high chiral separation selectivity
Inactive Publication Date: 2011-03-30
EAST CHINA UNIV OF SCI & TECH
View PDF3 Cites 11 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, this bonding meth
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
 Login to View More
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Login to View More
Abstract
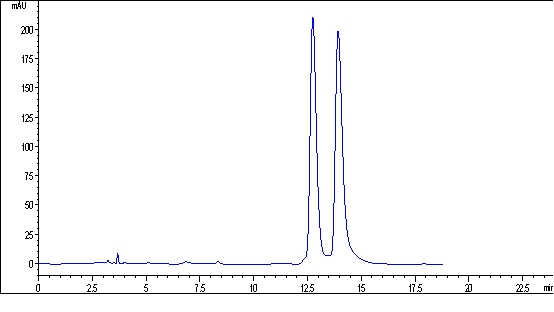
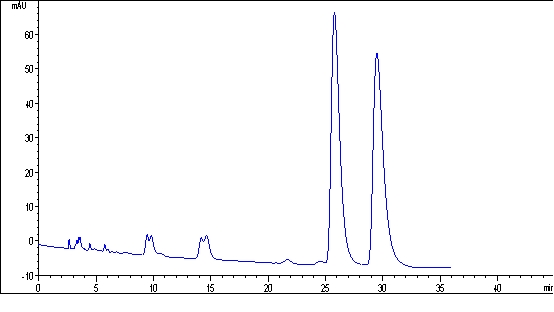
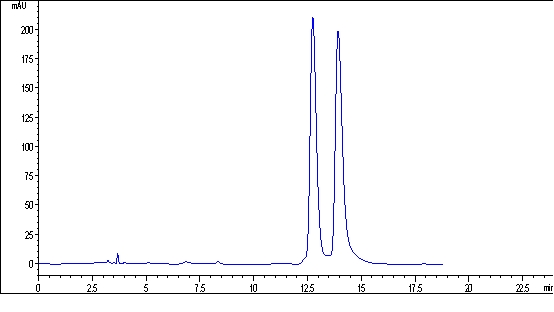
The invention discloses a chiral binaphthyl chromatogram immobile phase, and a preparation method and application thereof. The Chiral binaphthyl chromatogram immobile phase has a structural formula as shown in the accompanying drawing. The preparation method of the Chiral binaphthyl chromatogram immobile phase comprises the following steps that: the chiral binaphthyl reacts with 3-bromine alkynyl to generate alkynylation dinaphthol, and then the alkynylation dinaphthol reacts with 3-nitrine 3-aminopropyltriethoxy-silane through a clicking chemical method to obtain silane monomers containing chiral binaphthyl units; and the silane monomers are bonded onto the commercial silicon gel to obtain the immobile phase. The invention has the advantages that the structure of the immobile phase is novel, the preparation method is simple and has few steps, the last step of the monomer synthetization is the clicking chemical reaction, and the obtained chiral binaphthyl chromatogram immobile phase can be applied to the chiral binaphthyl chromatogram.
Description
technical field The invention relates to the field of chromatographic stationary phases of chemical technology, in particular to a chiral binaphthyl chromatographic stationary phase and its preparation method and application. Background technique Binaphthalene is a C2 axially asymmetric aromatic compound that has been studied more in recent years. It is easy to split into high-purity enantiomers, and has axial asymmetry and strong plane asymmetry. In addition, the two naphthalene rings on the binaphthyl molecule can provide a strong π-π force. For chromatographic stationary phases containing binaphthyl structures, past researches have mainly focused on the field of chiral chromatography. Among them, the binaphthyl crown ether chiral immobilizers have good resolution ability for chiral amino acids and chiral primary amines [Myung Ho H, Journal of Separation Science. 2006, 29(6), 750-761.]. Also, Oi.S [ Oi S, Shijo M, Tanaka H, Miyano S, Yamashita J, Journal of Chromatogra...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): B01J20/29B01J20/30G01N30/60
Inventor 梁鑫淼俞晖柯燕雄贾存宇余丹华许灵艳
Owner EAST CHINA UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com