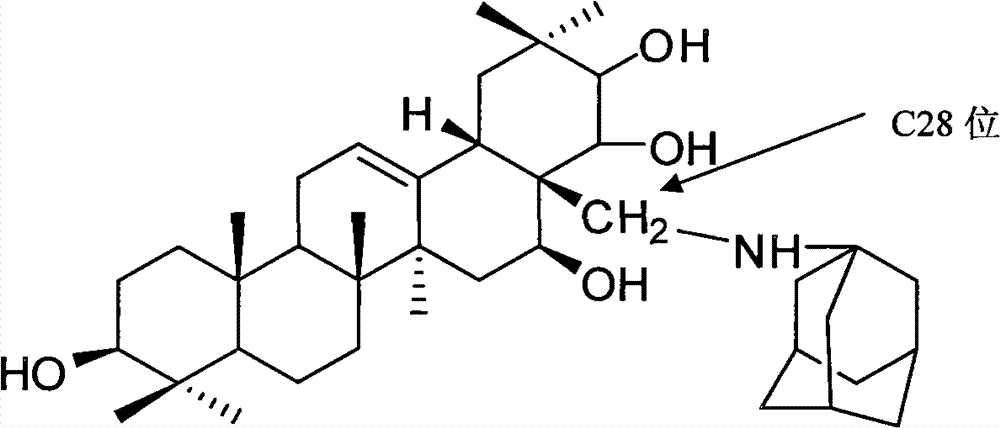
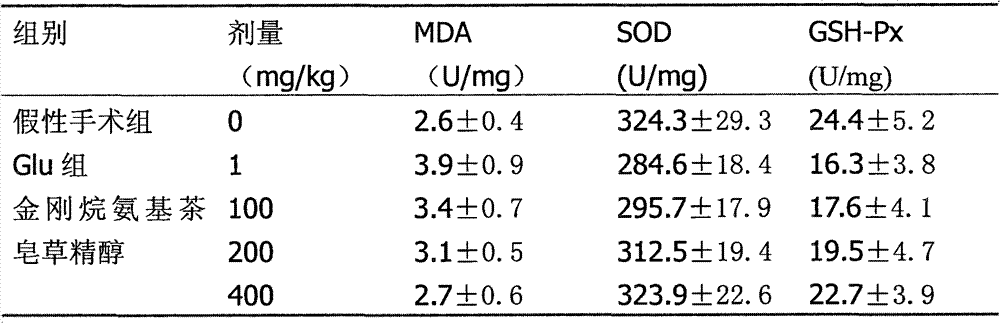
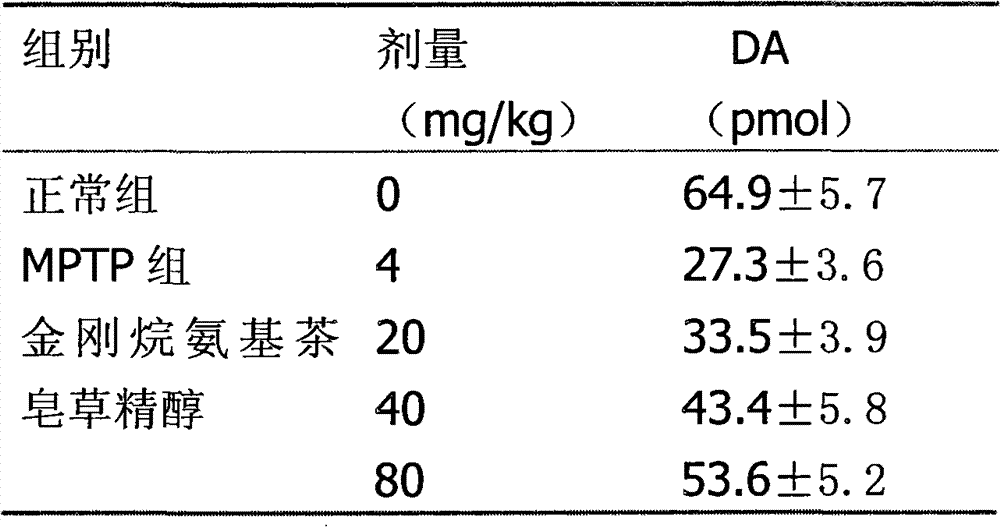
Adamantine amino tea sapogenol as well as preparation method and application thereof
A technology of adamantyl amino and tea soap, which is applied in the field of medicine, can solve the problems of mental side effects, decline and loss of curative effect, and achieve the effect of high product purity, simple preparation process and small structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 100g of teasapogenin solid into 300mL of pyridine, heat to 50°C to dissolve it; add triphenylchloromethane (56.7g) according to the equimolar amount of teasapogenin, keep warm at 50°C and stir for 8h; add teasapogenin 2 times the molar mass of acetic anhydride (41.6g), stirred and reacted at 50°C for 15h; added 9.4mL of formic acid, the reaction temperature was 100°C, and refluxed for 1h; after cooling to 20°C, added 45.6g of amantadine hydrochloride, triphenylphosphine 0.5g, 0.5g dimethyl azodicarboxylate, stir for 9h; add 56g of potassium carbonate, stir for 1h, evaporate pyridine under reduced pressure, add 1000mL water to wash, add toluene to wash the water-insoluble matter, and dissolve the residue with methanol and crystallized and dried in vacuo to yield 28 g of product.
[0034] The structural analysis of the product shows that MS: m / z 623; the elemental composition is: C77.00%, H10.50%, N2.24%, 010.26%, and its molecular formula is C 40 h 65 NO 4 , 1 H-...
Embodiment 2
[0036] Add 100g of teasapogenin solid into 300mL pyridine, dissolve it at room temperature (25°C), add triphenylchloromethane (85g) according to 1.5 times the molar mass of teasapogenin, keep warm at 25°C and stir for 12h; Acetic anhydride (83.2g) with 3 times the molar mass was stirred and reacted at 70°C for 10h; 14mL of formic acid was added, the reaction temperature was 105°C, and refluxed for 1h; after cooling to 25°C, 45.6g of amantadine, triphenylphosphine 10g, 10g of dimethyl azodicarboxylate, stirred for 6h; added 112g of potassium carbonate, stirred for 1h, evaporated pyridine under reduced pressure, added 1000mL of water to wash, then added toluene to wash, the residue was dissolved in methanol and crystallized, dried in vacuo , 45 g of product were obtained.
[0037] The analytical data of the product structure is the same as in Example 1, proving that the structure of the final product is as shown in Formula I.
Embodiment 3
[0039] Add 100g of teasapogenin solid into 300mL of pyridine, heat to 40°C to dissolve, add triphenylchloromethane (68g) according to 1.2 times the molar mass of teasapogenin, keep warm at 40°C and stir for 16h; add teasapogenin 4 times the molar mass of acetic anhydride (62.4g), stirred and reacted at 80°C for 8h; added 11.3mL of formic acid, the reaction temperature was 110°C, and refluxed for 1h; after cooling to 30°C, added 45.6g of amantadine, triphenylphosphine 3g, 3g of dimethyl azodicarboxylate, stirred for 3h; added 84g of potassium carbonate, stirred for 1h, evaporated pyridine under reduced pressure, added 1000mL of water to wash, then added toluene to wash, the residue was dissolved in methanol and crystallized, and dried in vacuo , 37 g of product were obtained.
[0040] The analytical data of the product structure is the same as in Example 1, proving that the structure of the final product is as shown in Formula I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com