Use of dronedarone or a pharmaceutically acceptable salt thereof, for the preparation of a medicament for use in regulating the potassium level in the blood
A technology of dronedarone and medicinal salts, applied in drug combinations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problem of lack of antiarrhythmic drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
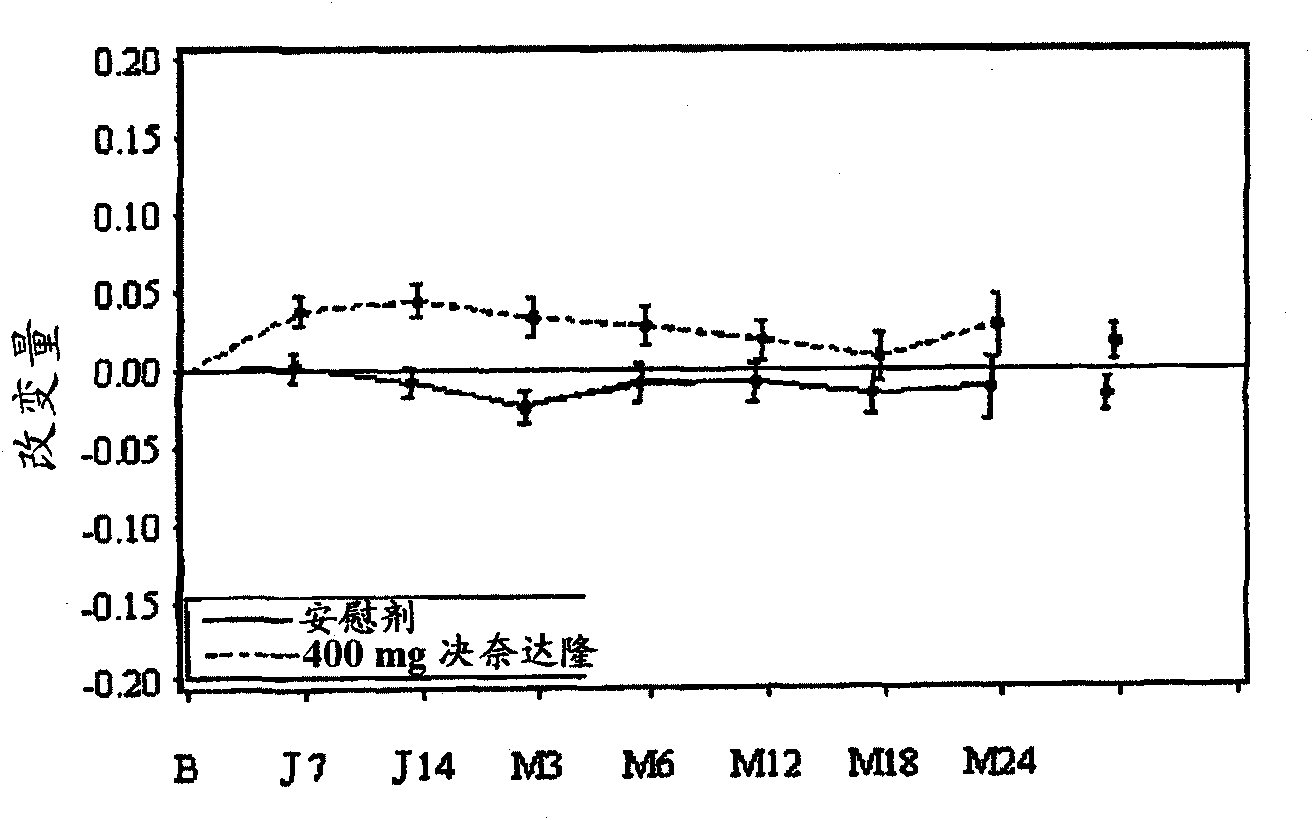
[0083] By dronedarone hydrochloride, in a prospective, multi-ethnic, multicenter, double-blind clinical study, two treatment groups (with dronedarone salt) in patients with a history of atrial fibrillation or atrial flutter The efficacy of dronedarone and its pharmaceutically acceptable salts compared to placebo in preventing cardiovascular hospitalization or preventing death was demonstrated.
[0084] I. Patient Selection
[0085] Eligible patients must have a history of atrial fibrillation or flutter and / or be able to exhibit normal sinus rhythm or atrial fibrillation or flutter.
[0086] The recruitment of patients considered the following selection criteria:
[0087] selection criteria :
[0088] 1) One of the following risk factors must exist:
[0089] - Age equal to or greater than 70, or even greater than 75, possibly combined with at least one of the following risk factors:
[0090] ○High blood pressure (taking at least two different types of antihypertensive dru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com