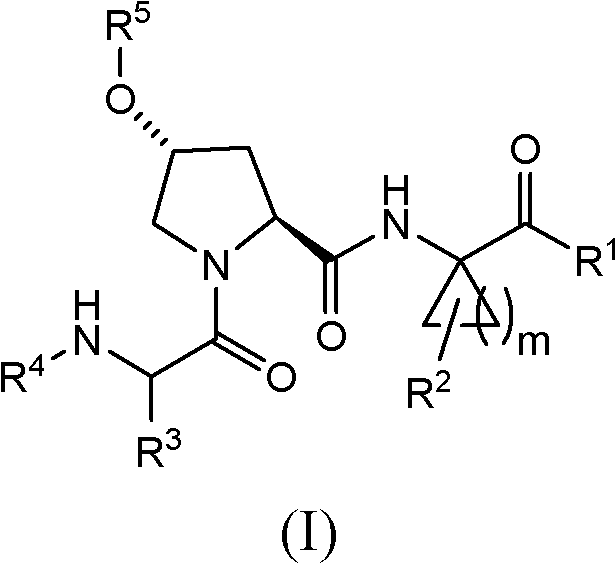
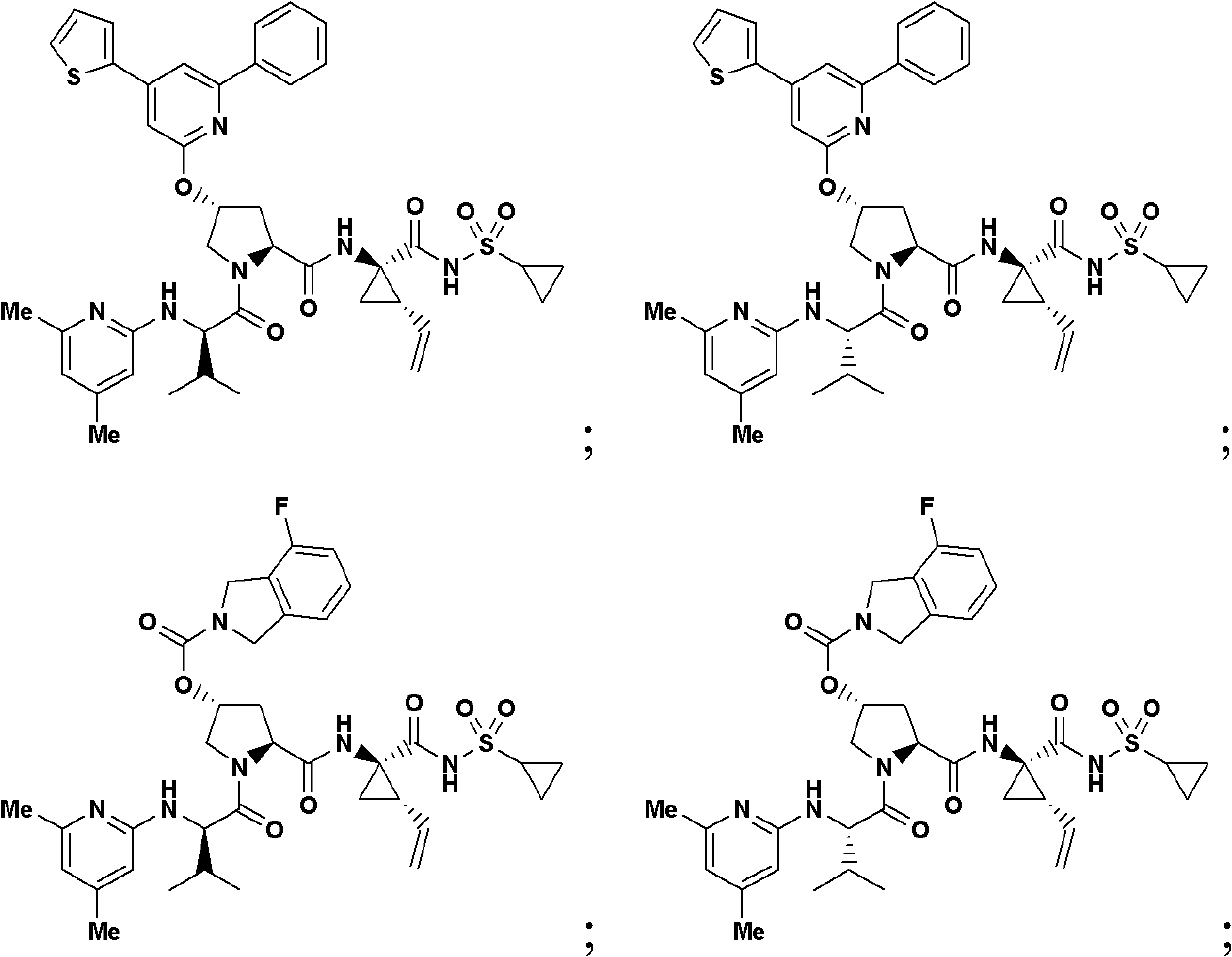
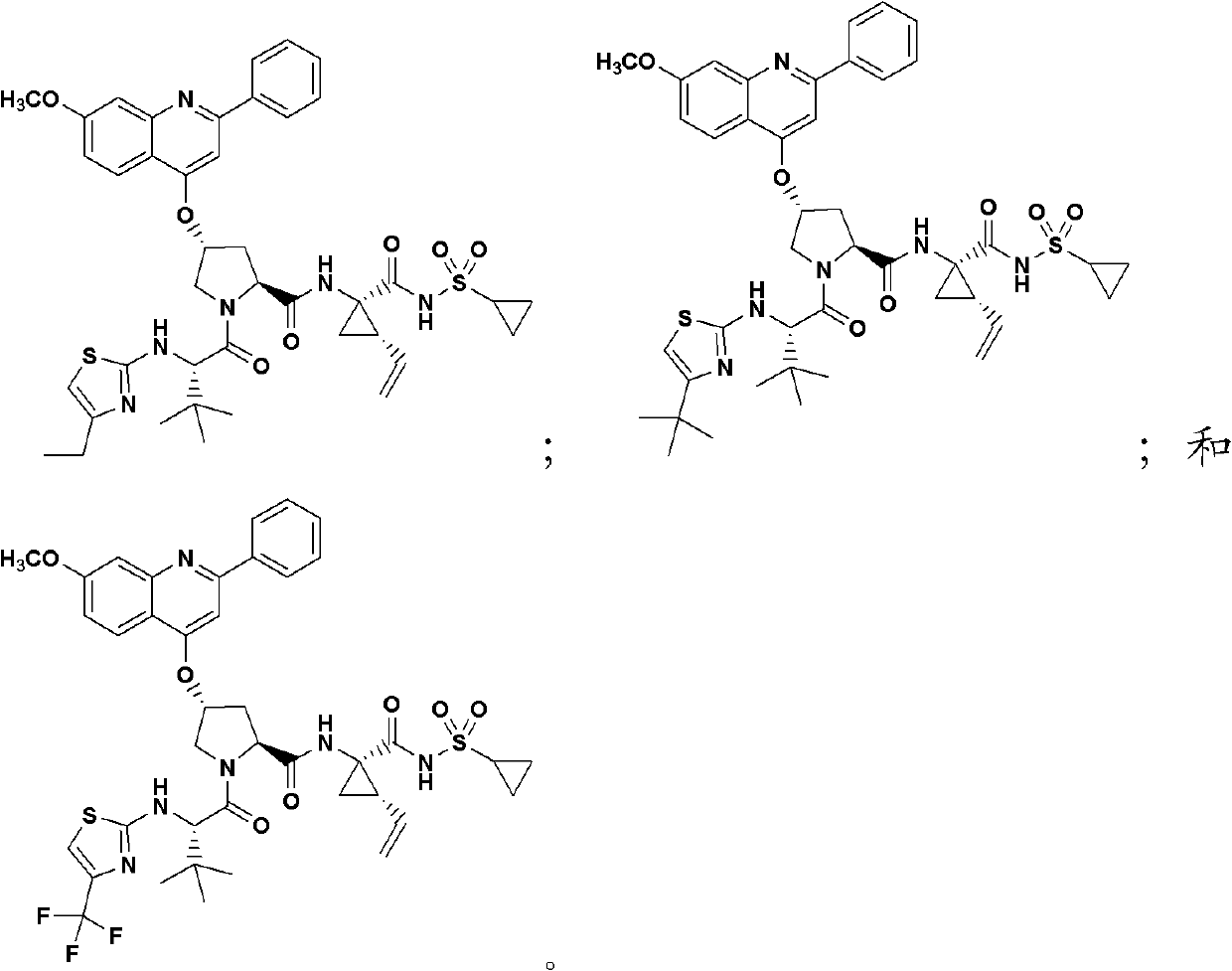
Hepatitis C virus inhibitors
An alkyl, selected technology, applied in the field of antiviral compounds, can solve problems such as viral load reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0286] Embodiment 1: the preparation of compound 1A and 1B
[0287]
[0288] plan 1
[0289]
[0290] step 1:
[0291] 6-Phenyl-4-(thiophen-2-yl)pyridin-2(1H)-one (1.07 mg, 4.23 mmol) (prepared according to S. Wang et al., Synthesis 4, 487-490, 2003) in A solution in phosphorus oxychloride (15 mL) was heated to reflux for three days. Excess phosphorus oxychloride was removed in vacuo and the residue was triturated with ice water. The trituration was basified with aqueous NaOH and the product was extracted into DCM. The organic layer was washed with brine, dried, filtered through celite and evaporated. The crude product was purified by flash column chromatography to give the product as a white solid (624 mg, 54% yield). 1 H NMR (CDCl 3 )δppm 7.16(dd, J=5.13, 3.7Hz, 1H), 7.44-7.52(m, 5H), 7.55(dd, J=3.7, 1.1Hz, 1H), 7.79(d, J=1.5Hz, 1H) , 8.02 (dd, J=8.1, 1.5Hz, 2H). LC-MS MS m / z 272 (M + +H).
[0292] Option 3
[0293]
[0294] Step 2:
[0295] To a solutio...
Embodiment 2
[0304] Embodiment 2: the preparation of compound 2
[0305]
[0306] Scenario 2
[0307]
[0308] step 1:
[0309] The product of step 1 of example 2 was prepared by the same procedure as the product of step 3 of example 1 starting from Boc-Hyp-OH instead of the product of step 2 of example 1 . 1 H NMR (500MHz, MeOD) δppm 1.09 (d, J = 7.63Hz, 2H) 1.16-1.22 (m, 1H) 1.25-1.32 (m, 1H) 1.42 (dd, J = 9.46, 5.49Hz, 1H) 1.47 ( s, 1.7H) 1.50 (s, 7.3H) 1.88 (dd, J = 8.09, 5.34Hz, 1H) 1.94-2.03 (m, 1H) 2.13 (dd, J = 12.97, 6.87Hz, 1H) 2.26 (q, J = 8.85Hz, 1H) 2.97 (ddd, J = 12.51, 8.09, 4.73Hz, 1H) 3.47 (d, J = 11.60Hz, 1H) 3.56-3.62 (m, 1H) 4.25 (dd, J = 9.61, 6.87 Hz, 1H) 4.42 (s, 1H) 5.15 (d, J = 10.38Hz, 1H) 5.34 (d, J = 17.09Hz, 1H) 5.74-5.85 (m, 1H). LCMS, MS m / z=442 (M-H) - .
[0310] Step 2:
[0311] To a solution of the product from Example 2, Step 1 (1.0 g, 2.25 mmol) in DCM (20 mL) was added 1,1'-carbonyldiimidazole (439 mg, 2.71 mmol). After stirring at ro...
Embodiment 3
[0318] Embodiment 3: the preparation of compound 3
[0319]
[0320] Scheme 1 of Example 3
[0321]
[0322] step 1:
[0323] To a solution of 3-methoxyaniline (300 g, 2.44 mol) and ethyl benzoyl acetate (234.2 g, 1.22 mol) in toluene (2.0 L) was added HCl (4.0 N in dioxane, 12.2 mL, 48.8 mmol). The resulting solution was refluxed for 6.5 hours using a Dean-Stark apparatus (approximately 56 mL of aqueous solution was collected). The mixture was cooled to room temperature, partitioned several times with aqueous HCl (10%, 3 x 500 mL), aqueous NaOH (1.0 N, 2 x 200 mL) and water (3 x 200 mL), and the organic layer was dried (MgSO 4 ), filtered and concentrated in vacuo to give an oily residue (329.5 g). The crude product was heated in an oil bath (280° C.) for 80 minutes using a Dean-Stark apparatus (approximately 85 mL of liquid was collected). The reaction mixture was cooled to room temperature, and the solid residue was washed with CH 2 Cl 2 (400 mL), the resulting...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com