High-safety medicinal composition of cinepazide, and preparation method and application thereof
A kind of technology of cinepazide and composition, which is applied in the field of cinepazide pharmaceutical composition and preparation thereof, and can solve the problems such as ineffective reduction and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The preparation of embodiment 1 cinepazide nitrogen oxide
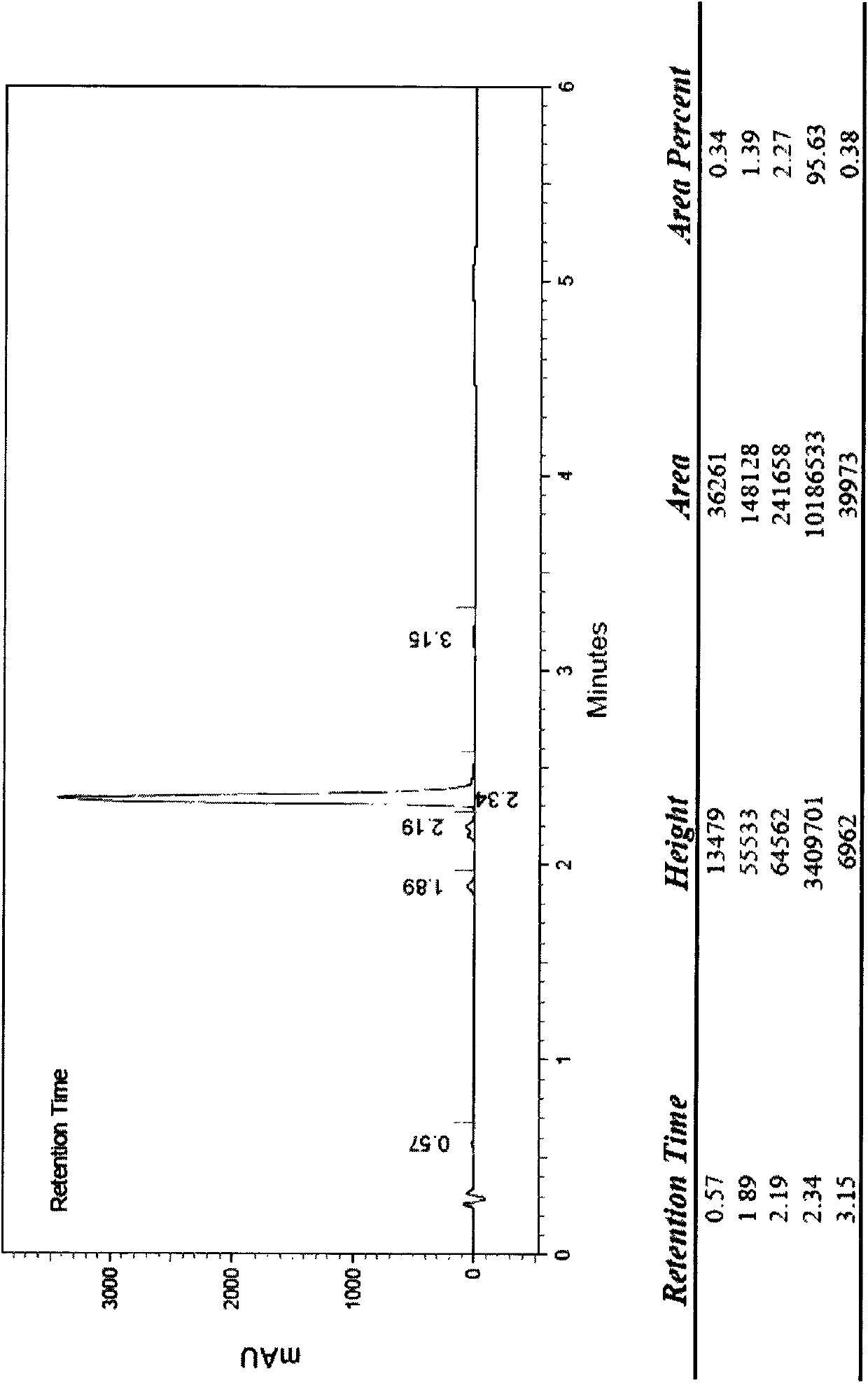
[0068] Dissolve 2 grams of cinepazide in 100 ml of dichloromethane, add 10 grams of peracetic acid, stir until the reaction is complete, filter, spin the filtrate, recrystallize with ethanol, and then freeze-dry to obtain 0.52 g of cinepazide special nitrogen oxides. HPLC peak area normalization method, the measured content is not less than 98%.
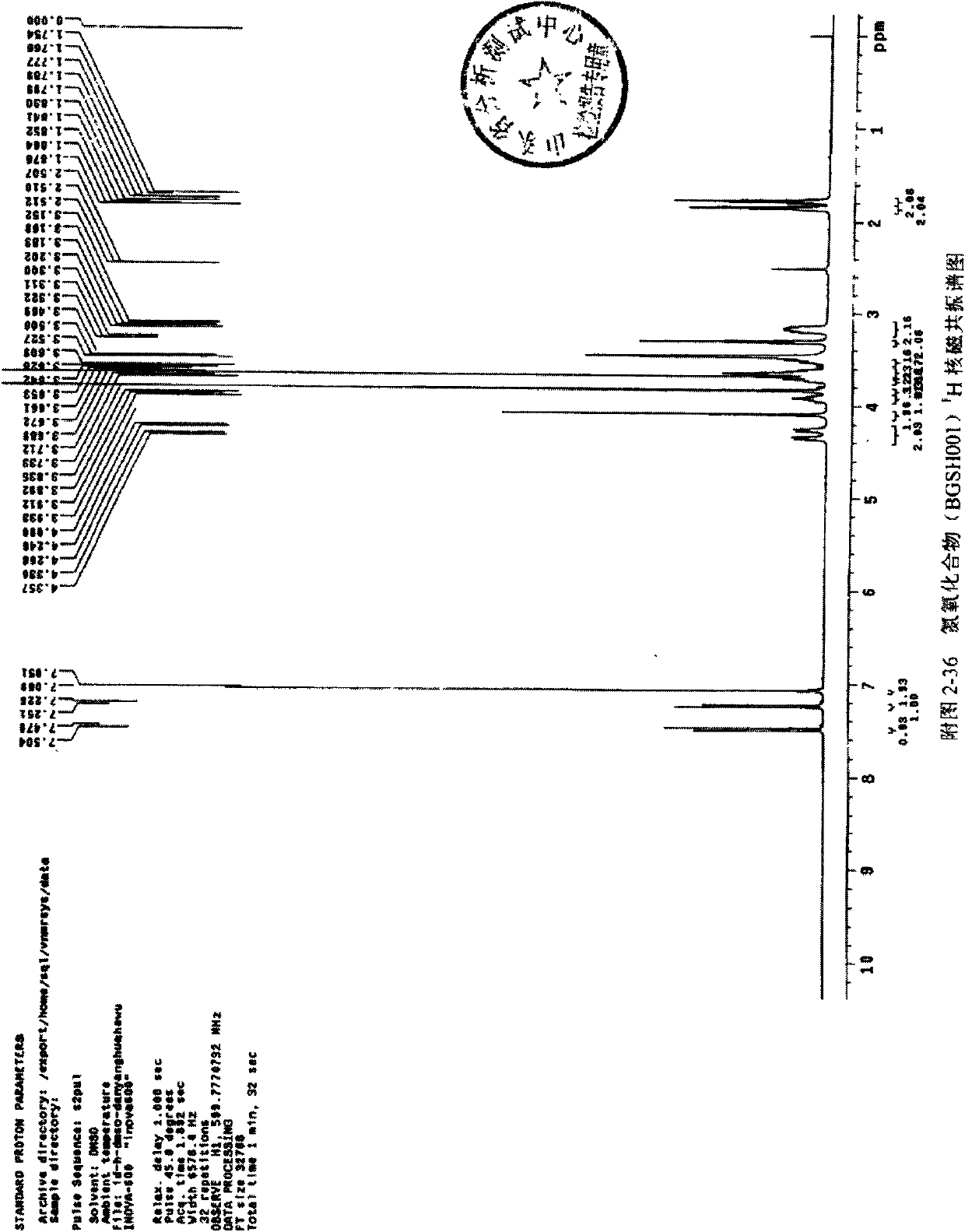
[0069] 1 H NMR (300MHz, CDCl 3 )δ (ppm): 7.615 (d, 1H) 6.731 (d, 1H) 6.610 (s, 2H) 3.801 (m, 1H), 4.012 (m, 2H), 4.131 (m, 1H) 4.674 (d, 1H) , 4.278(d,1H)3.907(s,6H)3.882(s,3H)3.251(d,2H),3.589(d,2H)3.504(t,2H)4.137(t,2H)1.914(m,2H) , 2.015(m, 2H)
Embodiment 2
[0070] The preparation of embodiment 2 cinepazide nitrogen oxides
[0071]Dissolve 2 grams of cinepazide in 100ml of methanol, add 5 grams of peroxybenzoic acid, stir until the reaction is complete, filter, spin the filtrate, and use preparative high-efficiency liquid phase separation, mobile phase is methanol-water, gradient wash Take off and receive the product fraction to obtain 0.3 g of cinepazide nitrogen oxide. HPLC peak area normalization method, the measured content is not less than 99%.
[0072] 1H NMR (600MHz, DMSO) δ (ppm): 7.492 (d, 1H) 7.238 (d, 1H) 7.060 (s, 2H) 4.306 (dd, 2H), 4.090 (s, 2H), 3.913 (m, 1H) 3.835(s, 6H), 3.723(m, 1H) 3.689(s, 3H) 3.641(m, 3H), 3.489(s, 1H) 3.311(t, 2H) 3.186(m, 2H) 1.853(m, 2H) , 1.776(m, 2H)
Embodiment 3
[0073] The preparation of embodiment 3 cinepazide nitrogen oxides
[0074] Dissolve 5 g of cinepazide maleate in 25 ml of water, add 25 ml of 25% hydrogen peroxide, stir and react at room temperature for 5 days, use preparative high-efficiency liquid phase separation, mobile phase is acetonitrile-water, gradient elution, receive The fraction of the product obtained 500 mg of cinepazide nitrogen oxide. HPLC peak area normalization method, the measured content is not less than 99%.
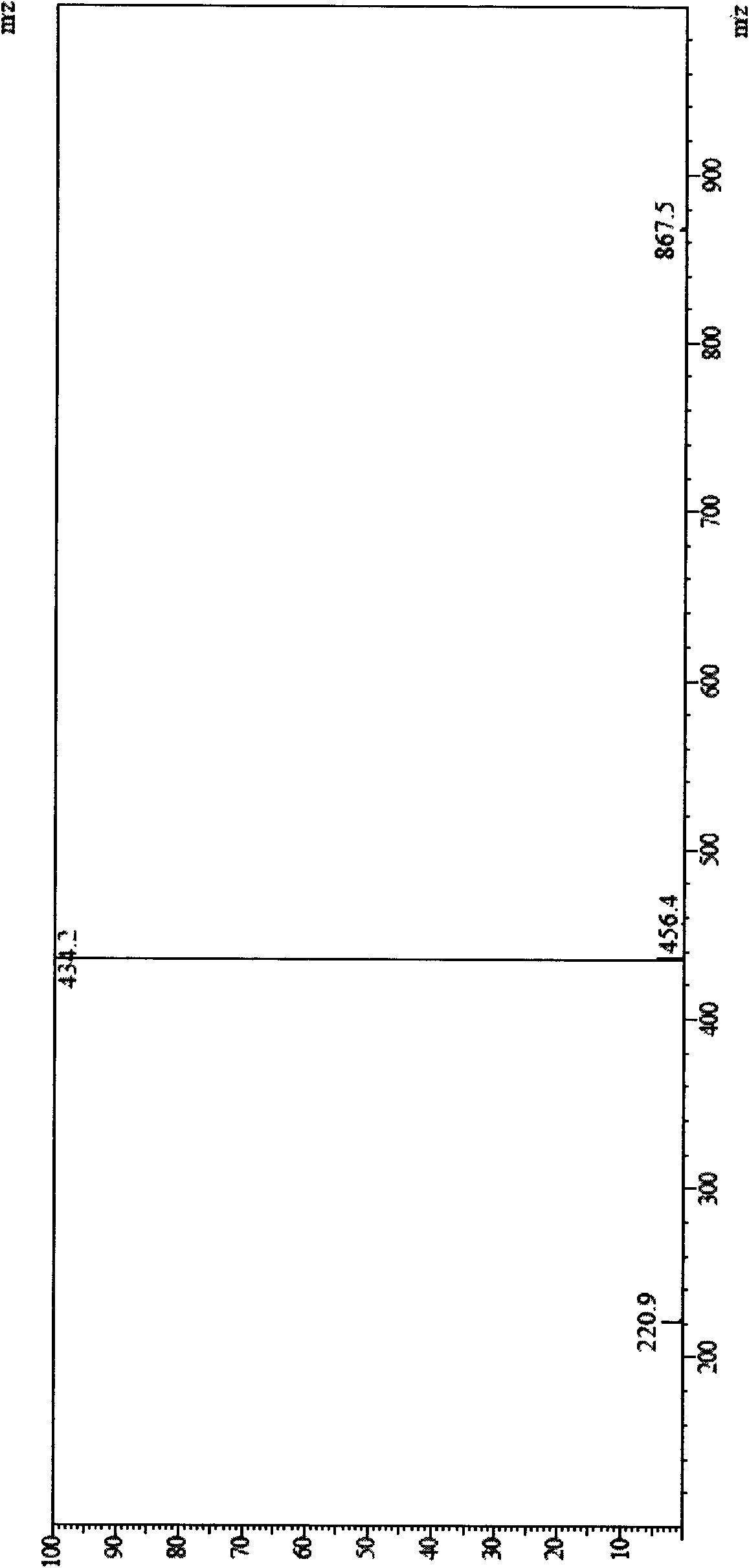
[0075] MS (LC-MS) = 434.2 (M+H + ), 867.5 (2M+H + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com