Crystal form I of clopidogrel hydrochloride and preparation method and application thereof
A technology of clopidogrel hydrochloride and clopidogrel, which is applied in the field of medicine and can solve problems such as poor reproducibility, low yield, and commercial scale-up
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
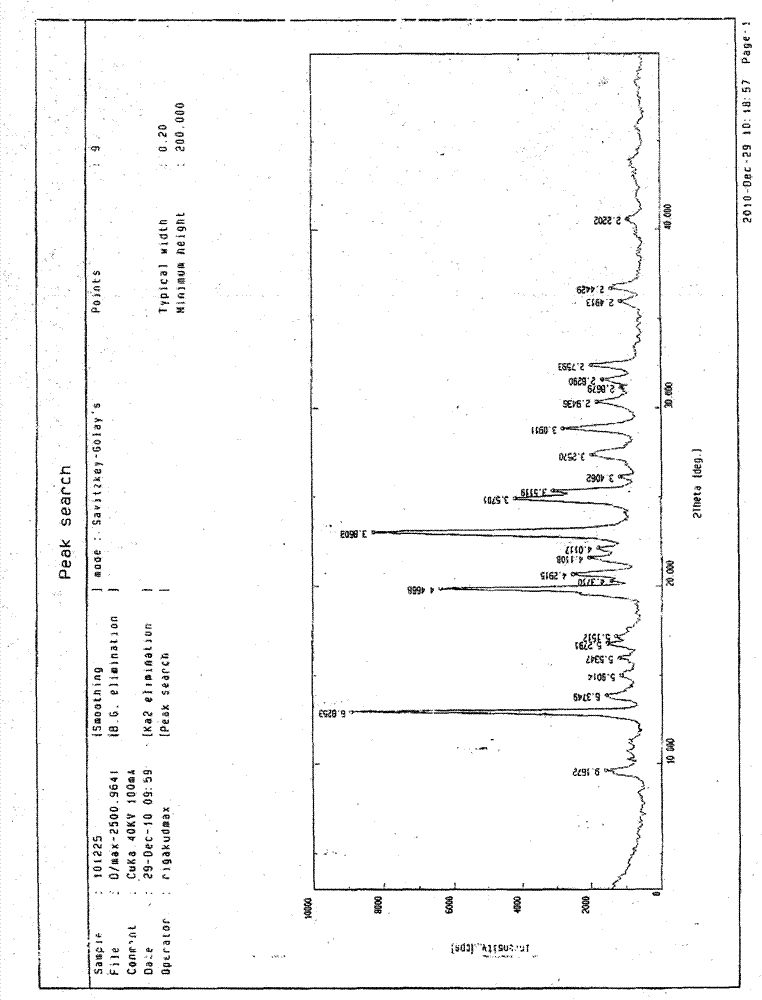
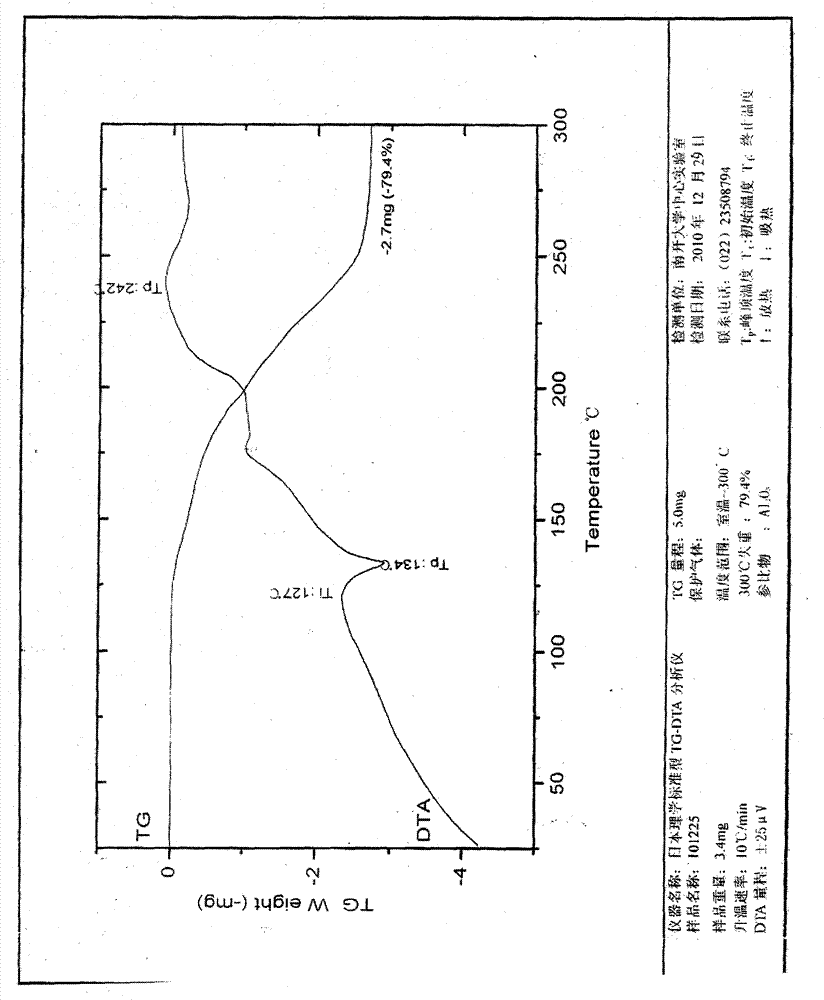
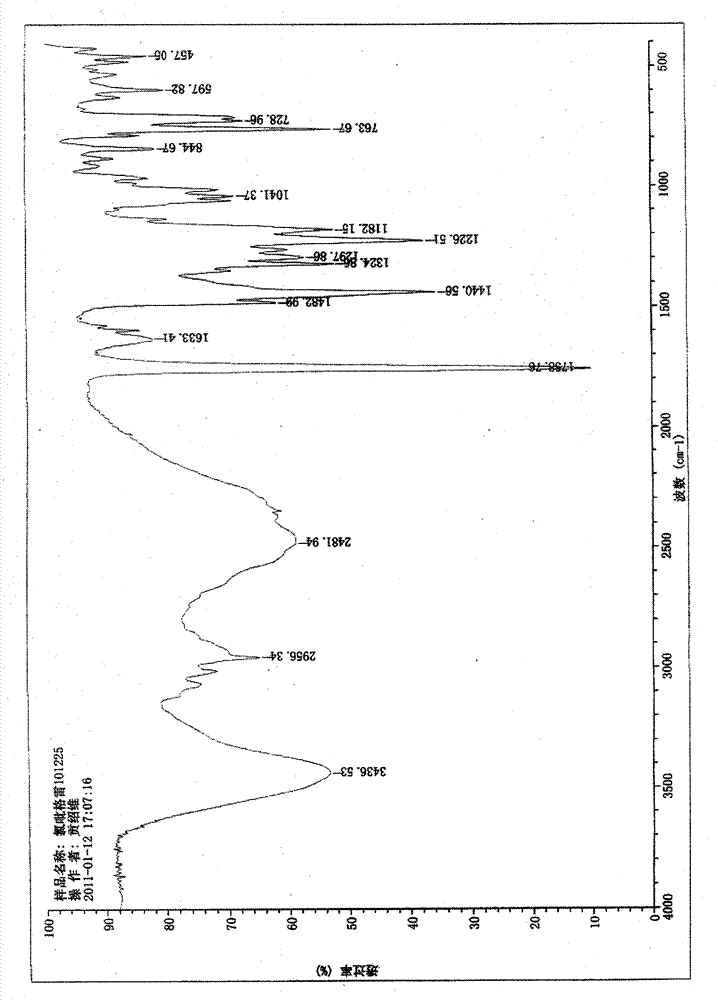
[0047] Example 1: Clopidogrel hydrochloride crystal form Ⅰ
[0048] Weigh 20.0g of clopidogrel, add it into 60mL of ethyl acetate, stir, dissolve all and mix evenly, cool to 5°C, add 5mol / L hydrochloric acid ethyl acetate solution dropwise, until no precipitation occurs, about 5ml, Maintain the temperature and stir for 4 hours, filter, and vacuum-dry for about 24 hours to obtain a white crystalline powder.
Embodiment 2
[0049] Example 2: Clopidogrel hydrochloride crystal form Ⅰ
[0050] Weigh 20.0g of clopidogrel, add it into 150mL of ethyl acetate, stir to dissolve all and mix evenly, cool to 5°C, add 5mol / L diethyl ether solution of hydrochloride dropwise until no precipitation occurs, about 5ml, keep the temperature Stir for 4 hours, filter, and vacuum dry for about 24 hours to obtain a white crystalline powder.
Embodiment 3
[0051] Example 3: Clopidogrel hydrochloride crystal form Ⅰ
[0052]Weigh 20.0g of clopidogrel, add it to 240mL of ethyl acetate, stir to dissolve all and mix evenly, cool to 5°C, add 5mol / L tert-butyl methyl ether hydrochloride solution dropwise until no precipitation occurs, about 5ml , kept stirring at the temperature for 4 hours, filtered, and dried in vacuum for about 24 hours to obtain a white crystalline powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com