Production process for preparing high-purity ApoA-I (Apolipoprotein A-I) from precipitates of plasma fraction IV
A technology of blood plasma component IV and buffer solution, which is applied in the production process field of preparing high-purity apolipoprotein ApoA-I, can solve the problems of loss of ApoA-I, unfavorable safety production, small preparation amount, etc., and achieve safe and convenient operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
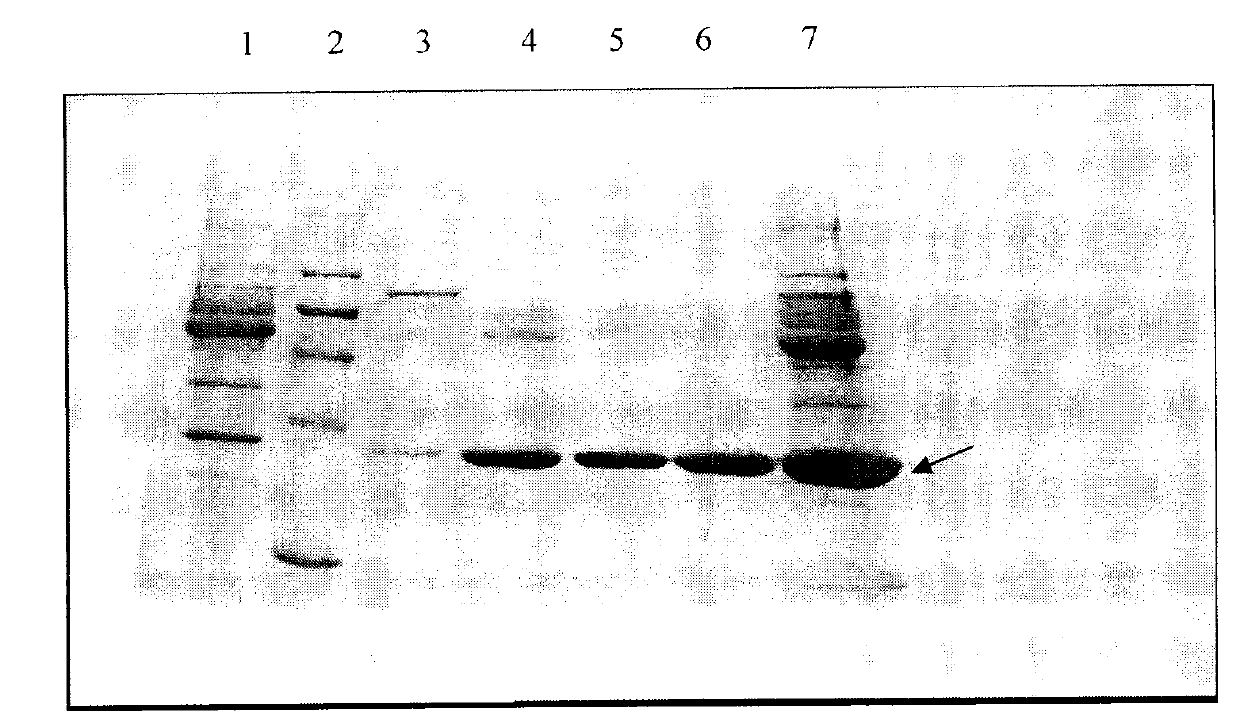
Examples
Embodiment 1
[0035] Example 1: ApoA-I sample preparation
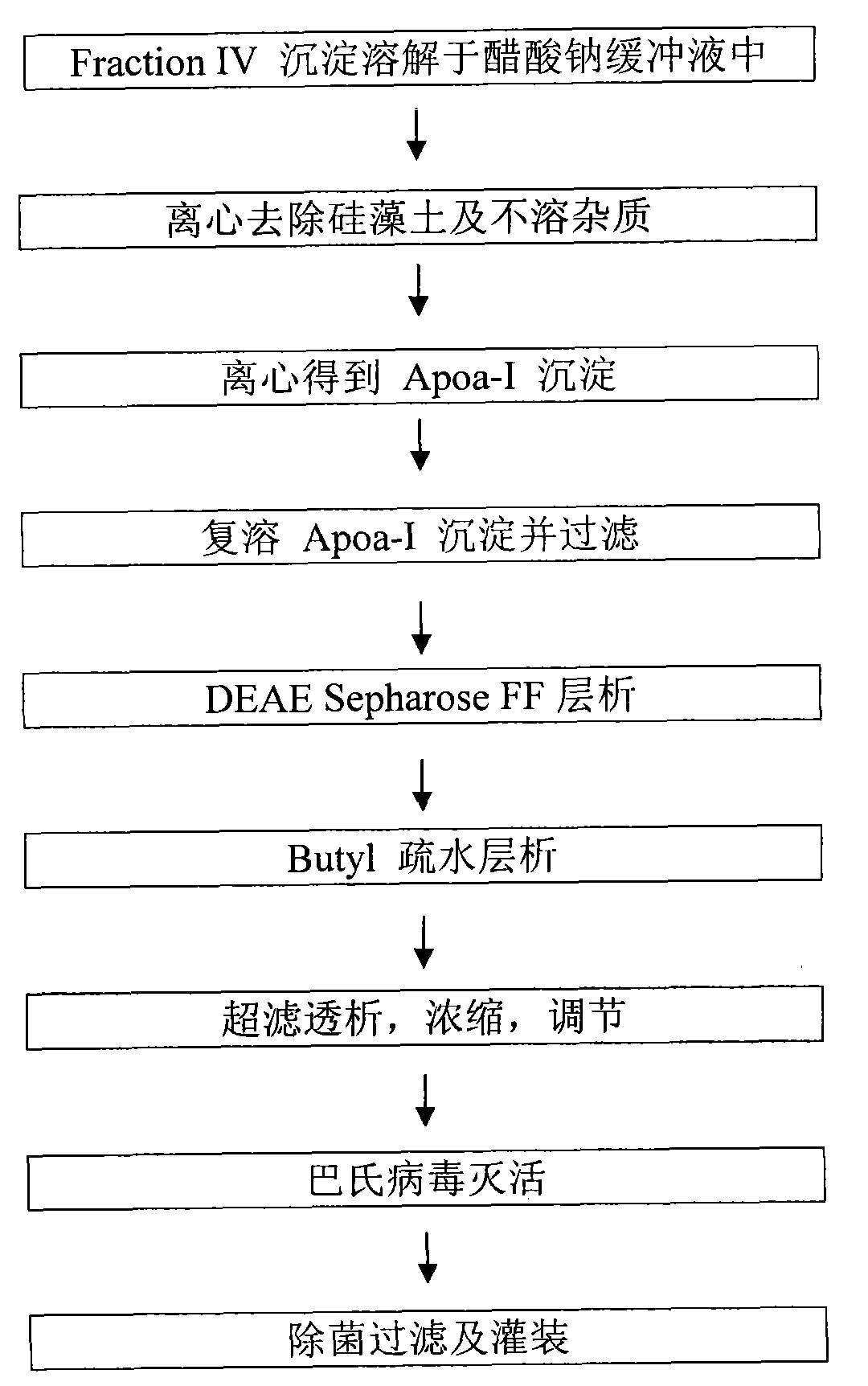
[0036] (1) Four precipitation dissolution and pretreatment of plasma components
[0037] Weigh 5 kg (wet weight) of component 4, dissolve it in 45 kg of sodium acetate buffer (control temperature is 0~8℃), adjust the pH to about 9.00, stir well to dissolve, and then centrifuge with Beckman The machine (7000rpm) removes diatomaceous earth and insoluble materials, and collects the centrifugal supernatant.
[0038] (2) Centrifugation to obtain ApoA-I precipitation
[0039] Sodium chloride is added to the supernatant to make the concentration reach about 2wt%, then the pH value is adjusted to 6.0-6.5, and the temperature is lowered to -1-1°C to make ApoA-I aggregate and precipitate. Centrifuge at high speed to collect about 500 g of ApoA-I precipitate, and discard the supernatant.
[0040] (3) Reconstituted ApoA-I precipitation
[0041] 500g of ApoA-I precipitate was fully dissolved in 5kg of 2wt% sodium chloride solution at about 5°C, and then...
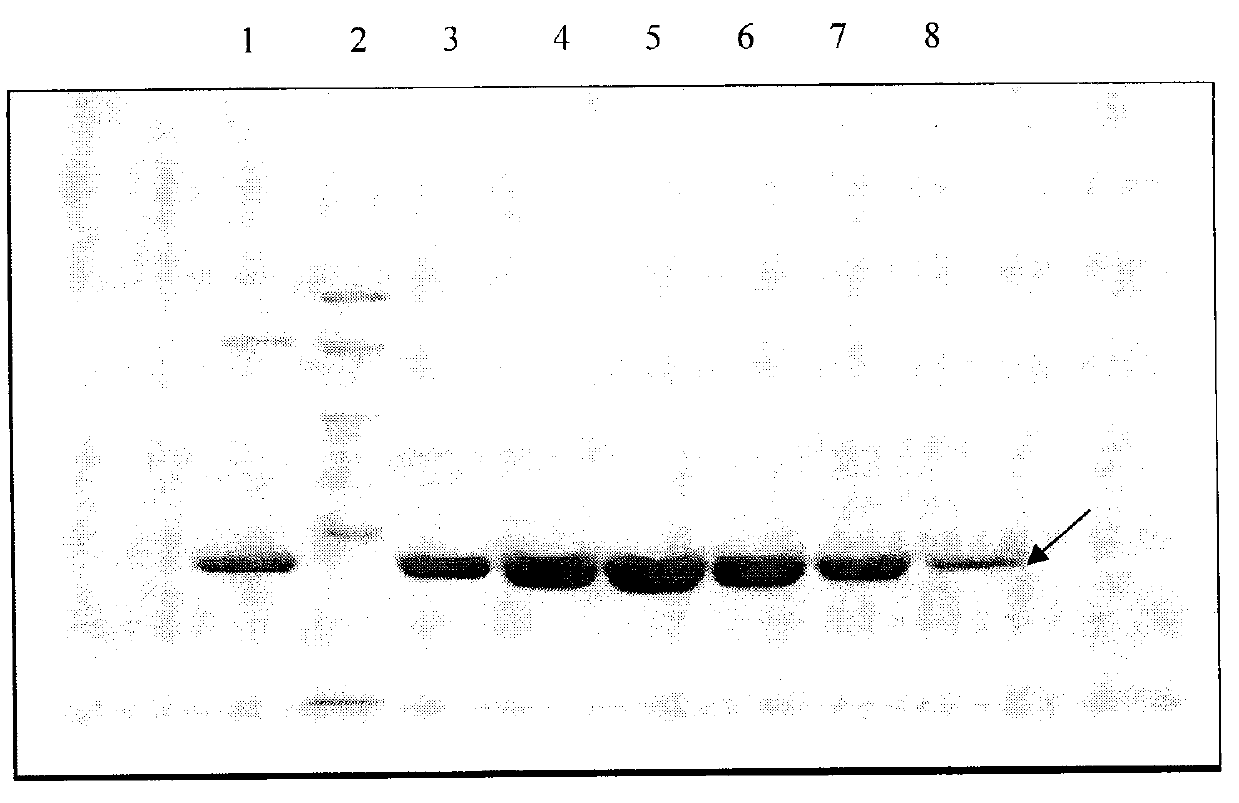
Embodiment 2
[0051] ApoA-I sample preparation
[0052] (1) Four precipitation dissolution and pretreatment of plasma components
[0053] Weigh 3 kg (wet weight) of component four, dissolve it in sodium acetate buffer (control the temperature at 0~8℃), adjust the pH to 8.00, stir well to dissolve it, and then centrifuge at 7000rpm to remove the diatomaceous earth and Insoluble matter, collect the centrifuged supernatant.
[0054] (2) Centrifugation to obtain ApoA-I precipitation
[0055] Sodium chloride was added to the supernatant to make the concentration reach 1wt%, then the pH value was adjusted to 6.0-6.5, and the temperature was lowered to -1-1°C to make ApoA-I aggregate and precipitate. Centrifuge at high speed to collect about 300 g of ApoA-I precipitate, and discard the supernatant.
[0056] (3) Reconstituted ApoA-I precipitation
[0057] 300 g of ApoA-I precipitate was fully dissolved in a 1 wt% sodium chloride solution at 0°C, and then filtered with a 0.45 μm filter membrane.
[0058] (4) ...
Embodiment 3
[0062] ApoA-I sample preparation
[0063] (1) Four precipitation dissolution and pretreatment of plasma components
[0064] Weigh 6 kg (wet weight) of component four, dissolve it in sodium acetate buffer (control temperature is 0~8℃), adjust the pH to 10.00, stir well to dissolve, and then centrifuge at 7000rpm to remove diatomaceous earth And insoluble matter, collect the centrifuge supernatant.
[0065] (2) Centrifugation to obtain ApoA-I precipitation
[0066] Sodium chloride was added to the supernatant to make the concentration reach 3wt%, then the pH value was adjusted to 6.0-6.5, and the temperature was lowered to -1-1°C to make ApoA-I aggregate and precipitate. Centrifuge at high speed to collect about 600 g of ApoA-I precipitate, and discard the supernatant.
[0067] (3) Reconstituted ApoA-I precipitation
[0068] Dissolve 600 g of ApoA-I precipitate in a 3 wt% sodium chloride solution at about 10° C., and then filter with a 0.45 μm filter membrane.
[0069] (4) Column chromato...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com