Photochromic 2h-chr0menes annulated at c5-c6 and their methods of preparation
A cycloalkyl, straight-chain technology, applied in the field of photochromic C5-C6 cyclic 2H-chromene and its preparation, to achieve the effect of increasing the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0070] The methods described herein will be specifically described in conjunction with various embodiments. The following examples are not intended to limit the invention, but are illustrative of its implementation. Efforts have been made to ensure accuracy with respect to numbers (eg, amounts, temperature, etc.), but some error or deviation may occur. Unless indicated otherwise, parts are parts by weight, temperature is in °C or is at ambient temperature, and pressure is at or near atmospheric.
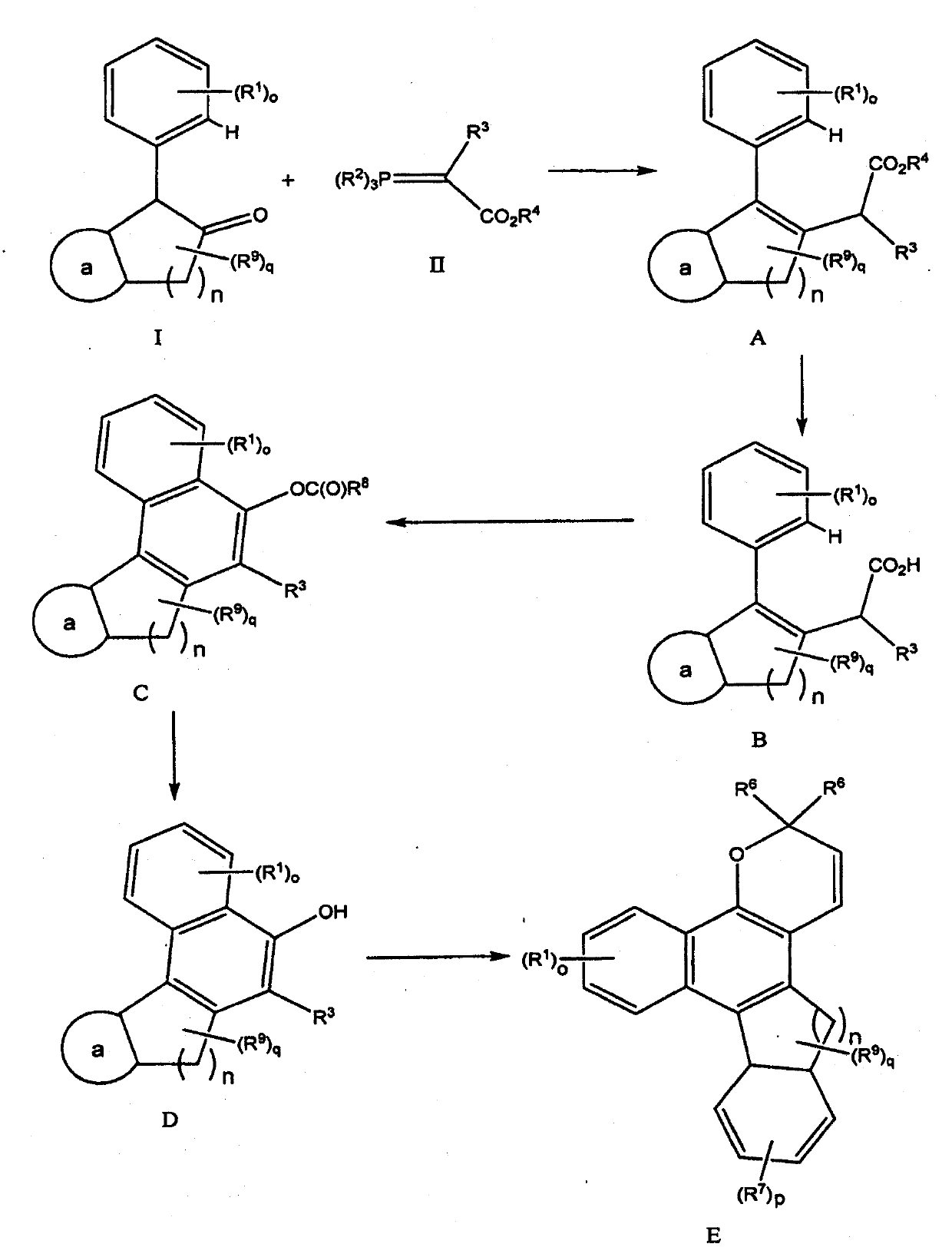
[0071] I. Preparation of C using the method described herein 5 -C 6 Cyclic naphthopyrans
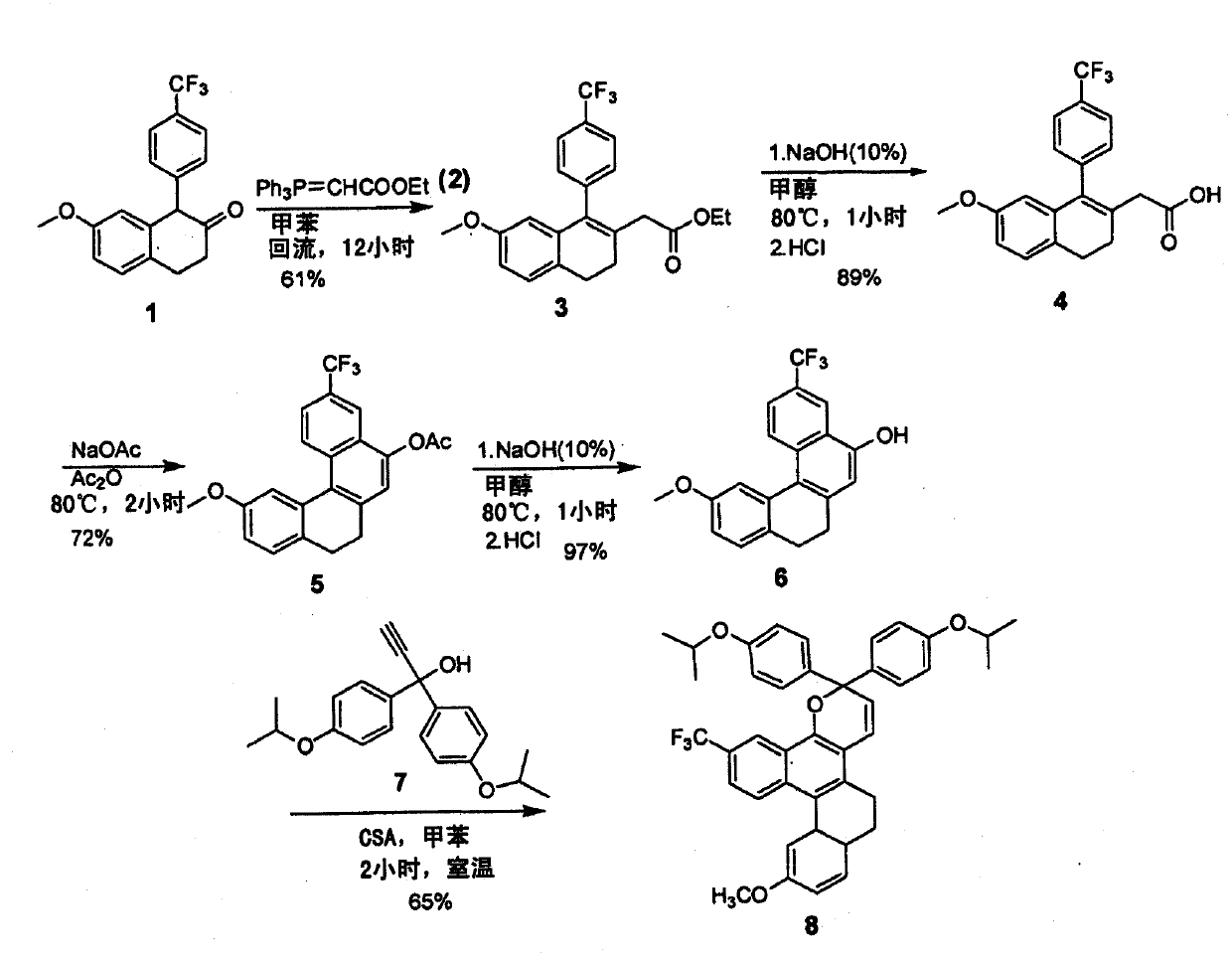
[0072] figure 2 All numbers mentioned below are shown in . Table 1 provides the reaction yields for four different series of compounds Steps 1-5.
[0073] Step 1: Wittig Reaction
[0074]
[0075] Ketone 1 (1.7 g, 5.3 mmol) and Wittig ylide 2 (3.7 g, 10.6 mmol) were placed in dry toluene (20 mL ), reflux at 130°C (oil bath temperature) for 12 hours. The toluene was then removed in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com