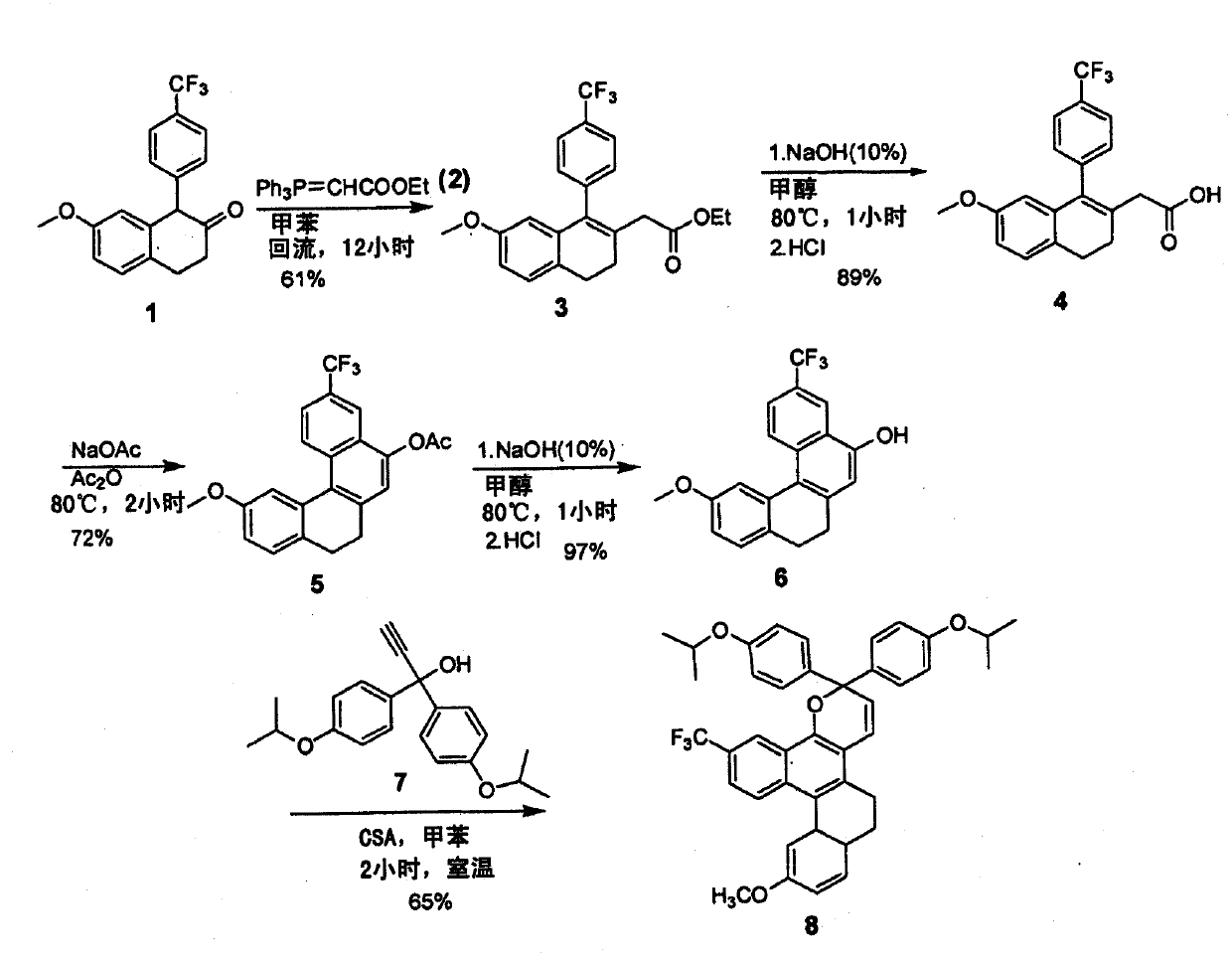
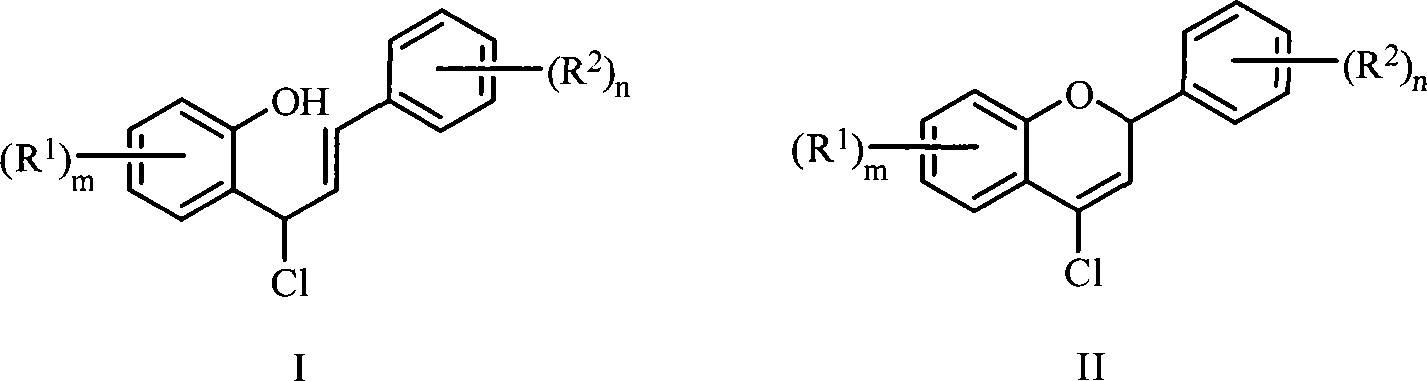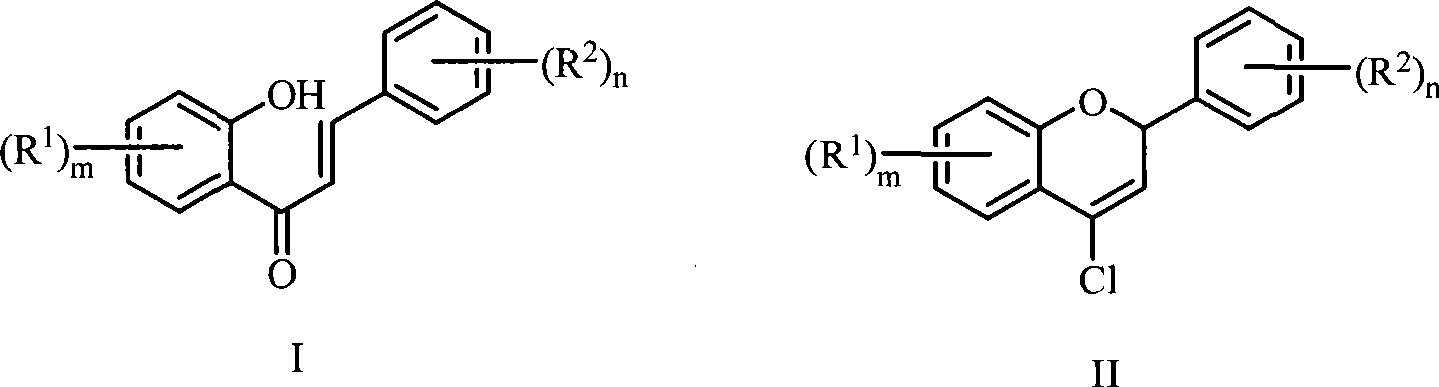Patents
Literature
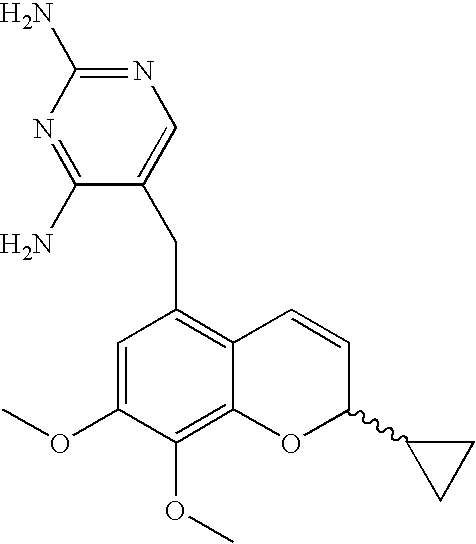
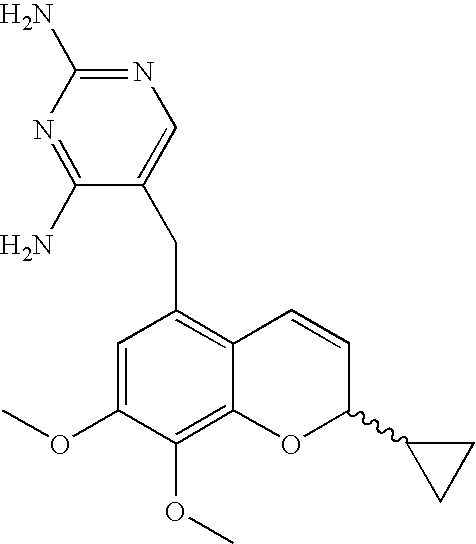
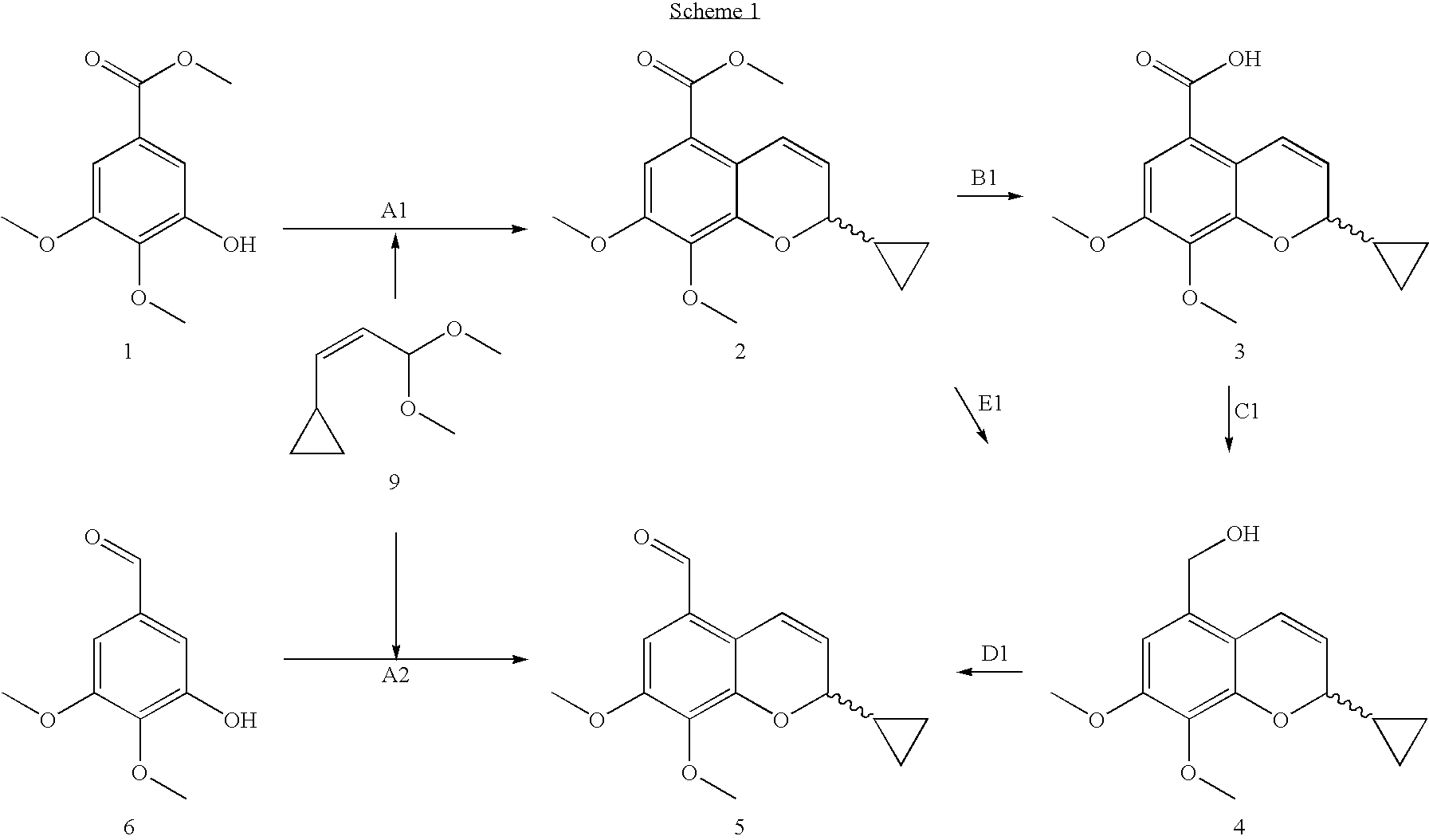
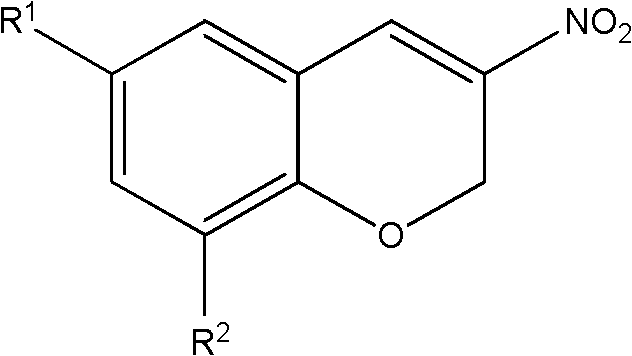
44 results about "2H-chromene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
2H-chromene is a simplest member of the class of chromene in which the heterocyclic pyran ring has a double bond between positions 3 and 4. It is a chromene and an organic heterobicyclic compound. It is a tautomer of a 4H-chromene.
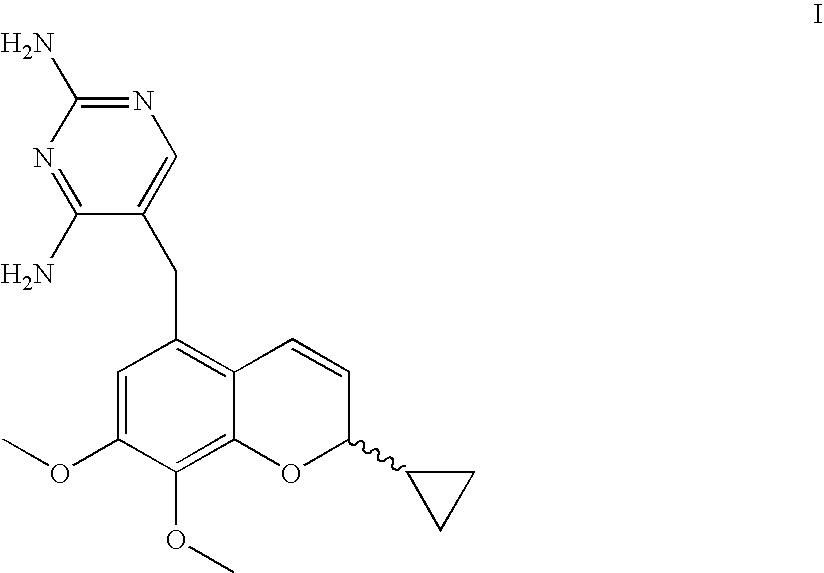
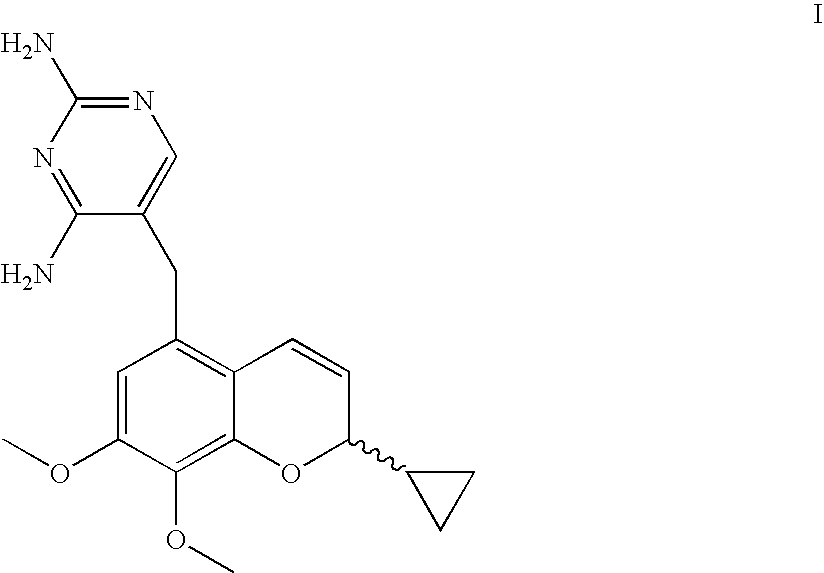
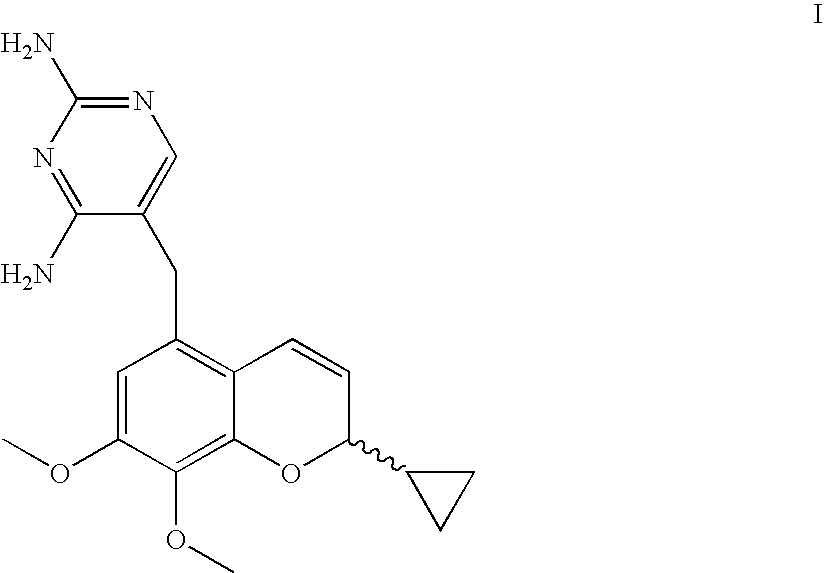
Novel Benzopyran Compounds, Compositions and Uses Thereof
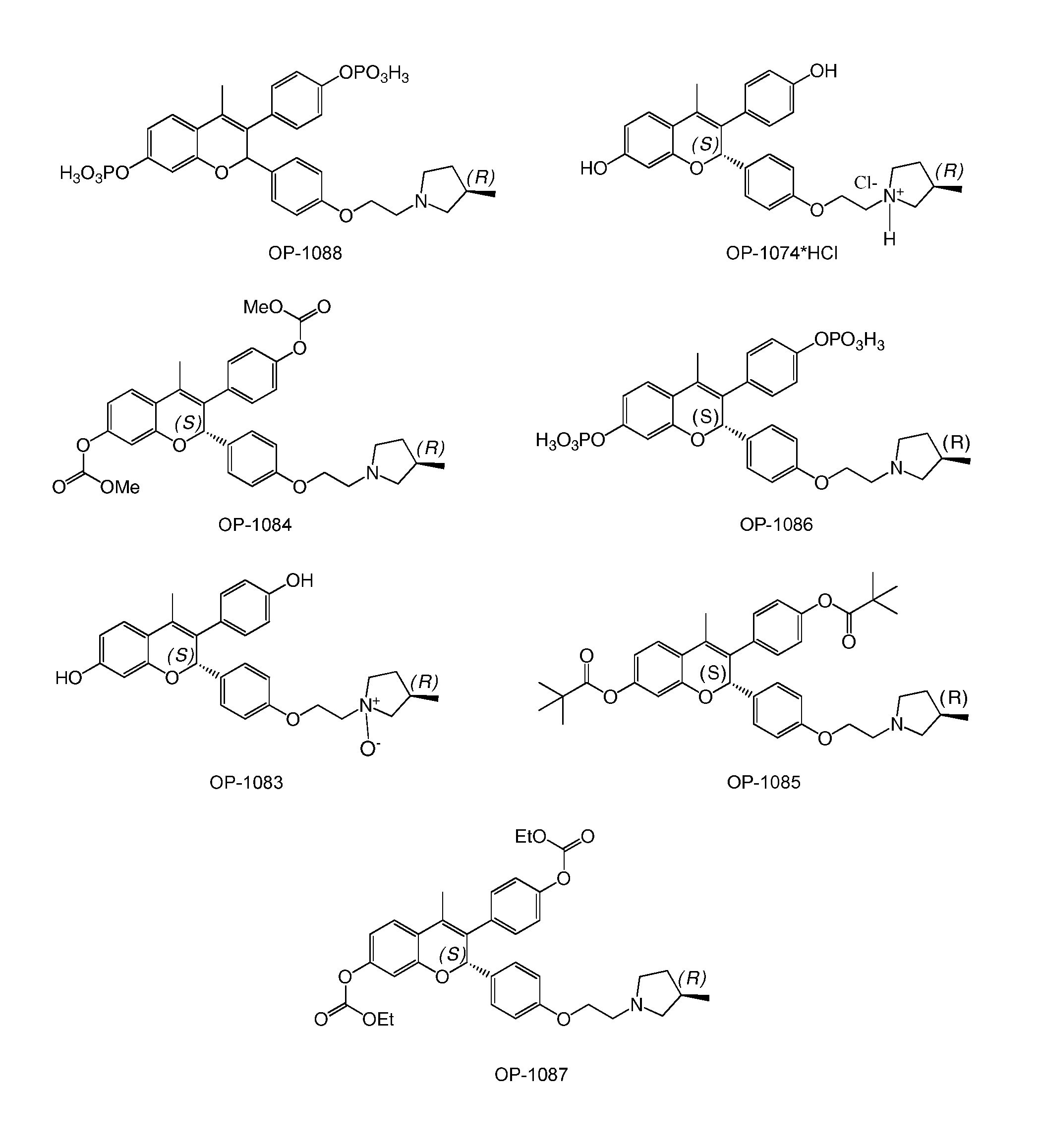
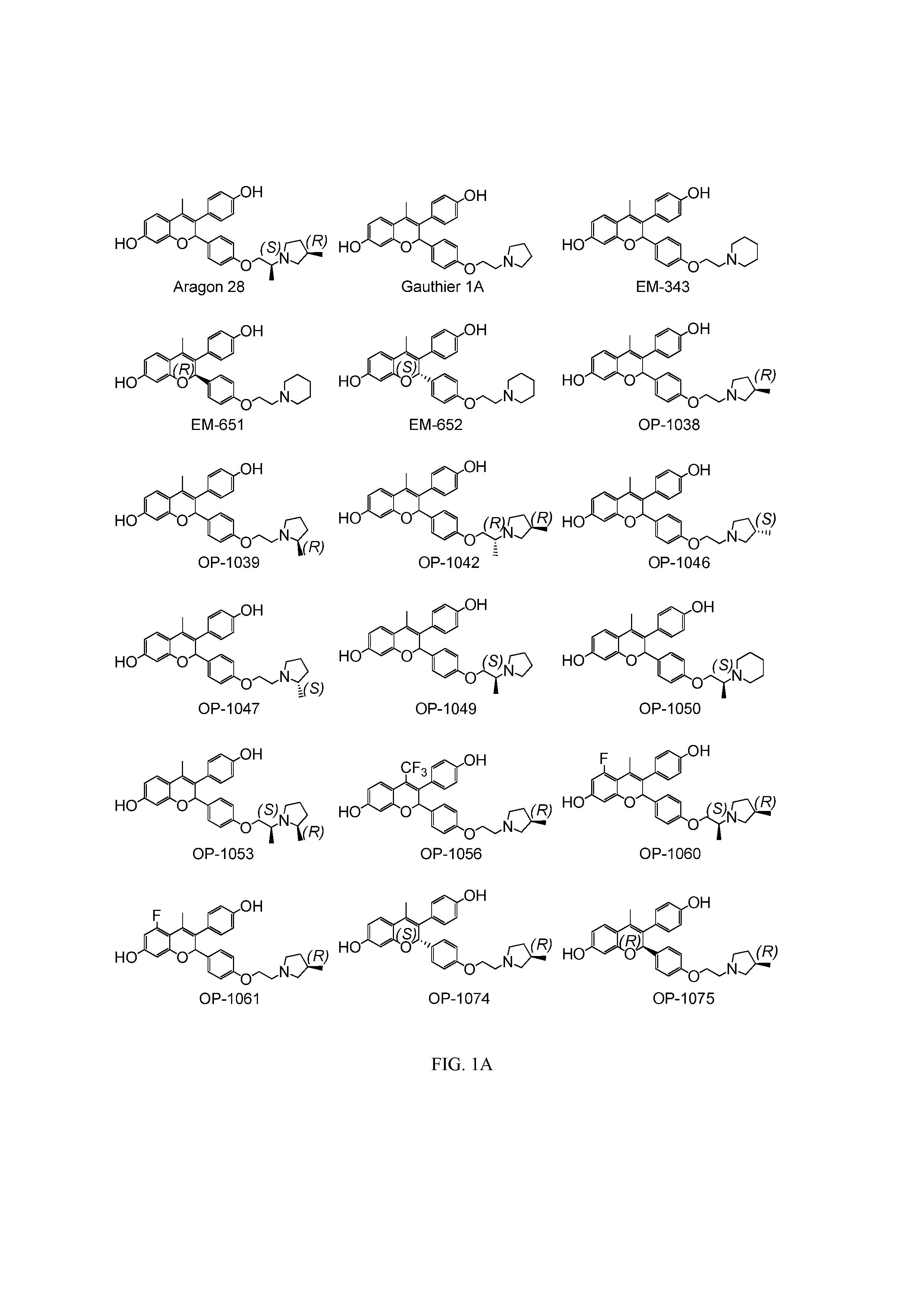
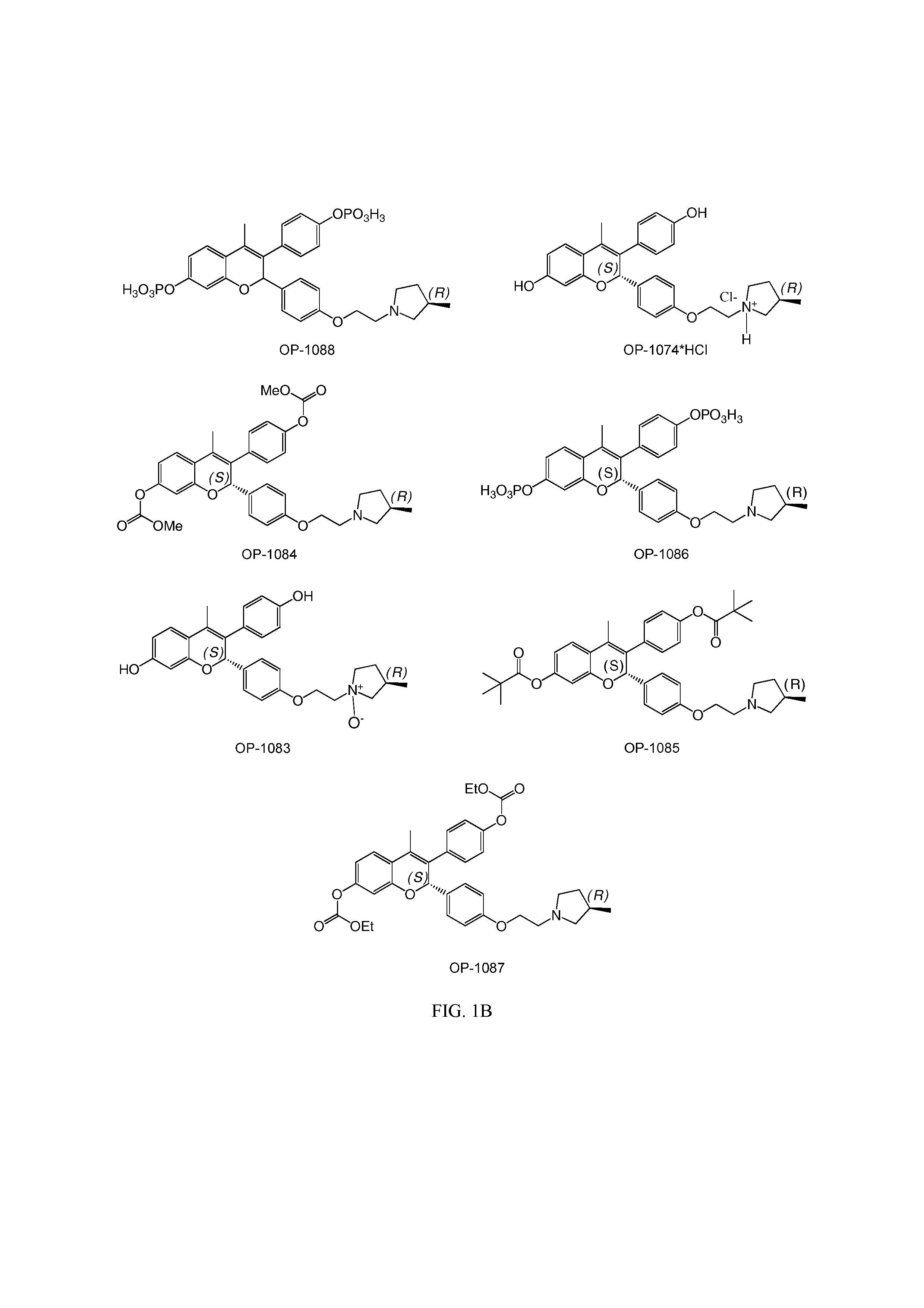
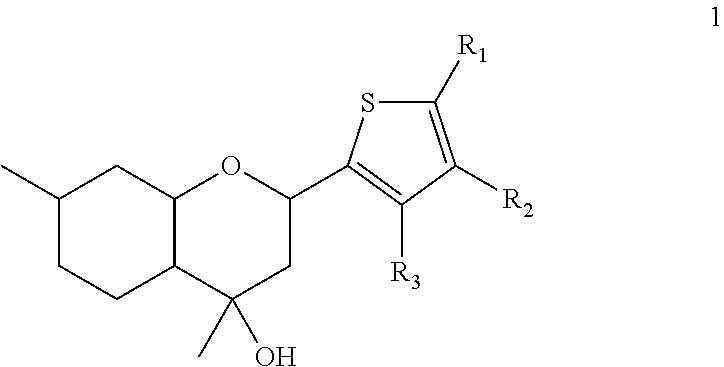
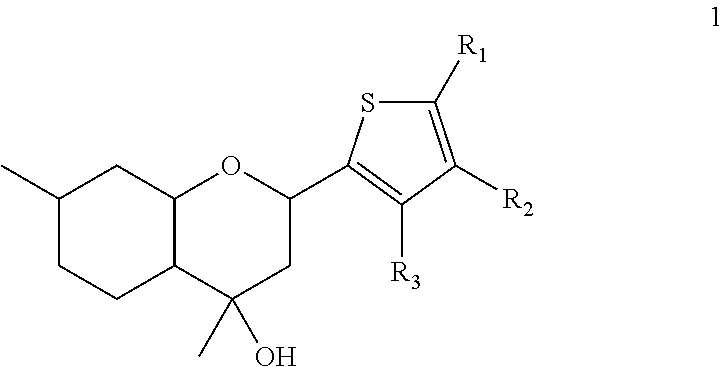
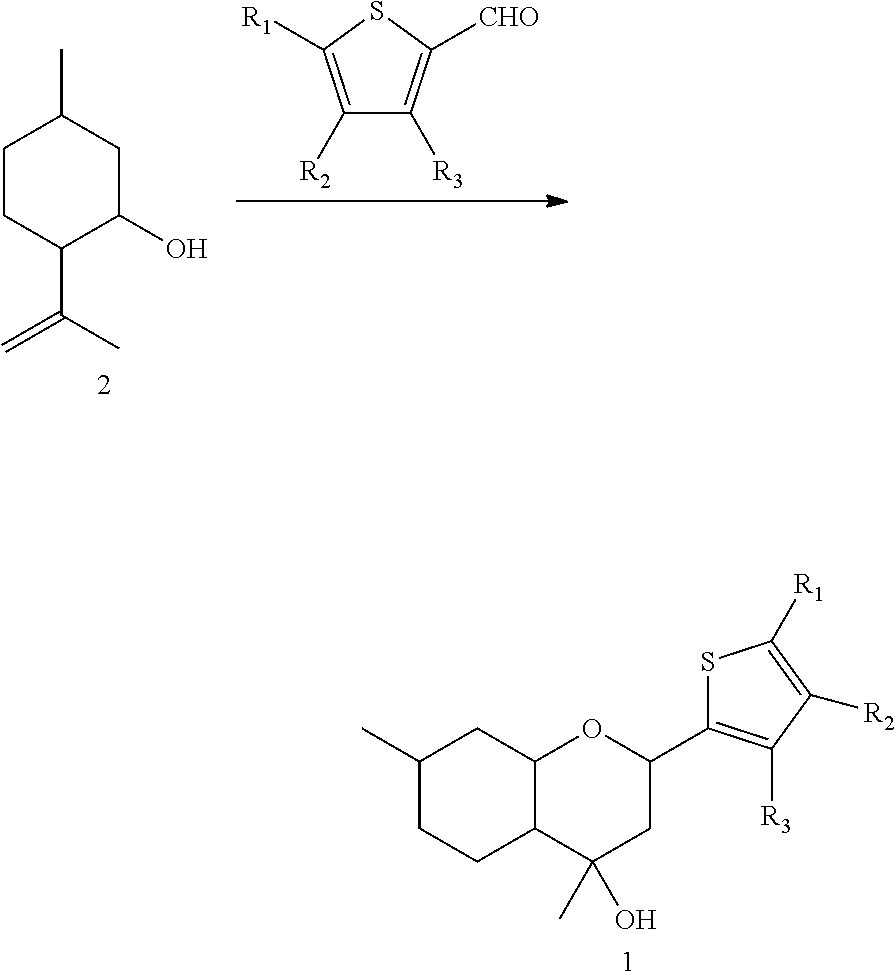
Benzopyran compounds with strong anti-estrogenic activity and essentially no estrogenic activity are provided, which are OP-1038, which is 3-(4-hydroxyphenyl)-4-methyl-2-(4-{2-[(3R)-3-methylpyrrolidin-1-yl]ethoxy}phenyl)-2H-chromen-7-ol, and OP-1074, which is (2S)-3-(4-hydroxyphenyl)-4-methyl-2-(4-{2-[(3R)-3-methylpyrrolidin-1-yl]ethoxy}phenyl)-2H-chromen-7-ol. OP-1074 is a pure anti-estrogen when tested in the agonist mode and a complete anti-estrogen when tested in the antagonist mode. These compounds are useful for the treatment or prevention of a variety of conditions that are modulated through the estrogen receptor in mammals including humans.
Owner:OLEMA PHARMA
Substituted 4h-chromens, 2h-chromenes, chromans and analogs as activators of caspases and inducers of apoptosis and the use thereof
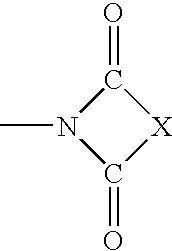
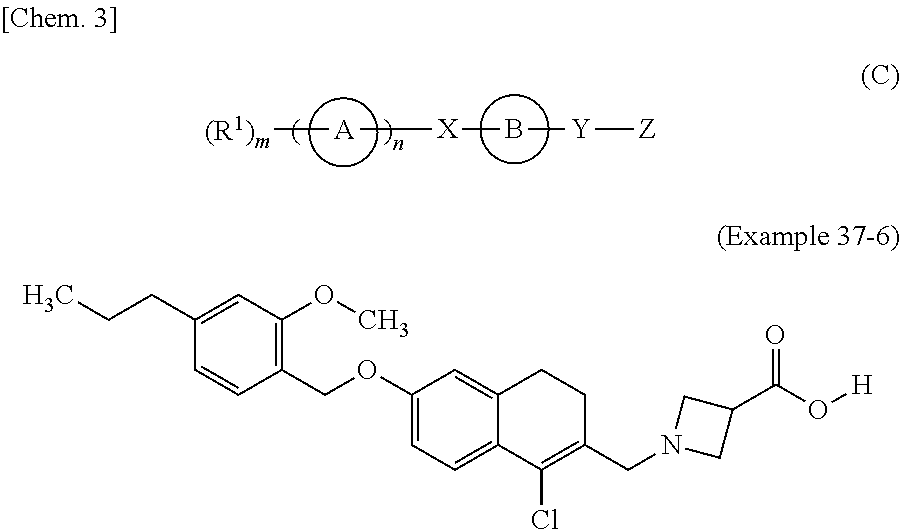
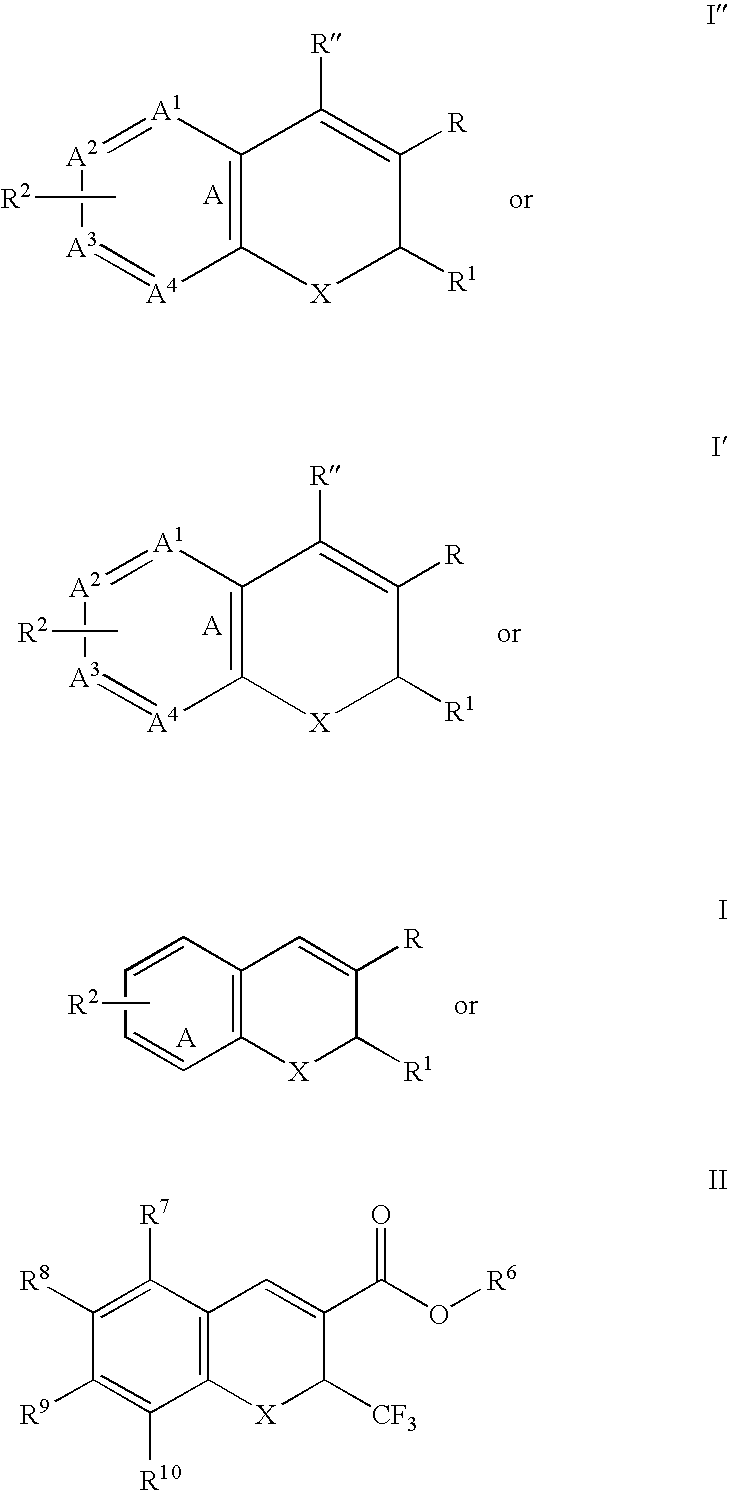
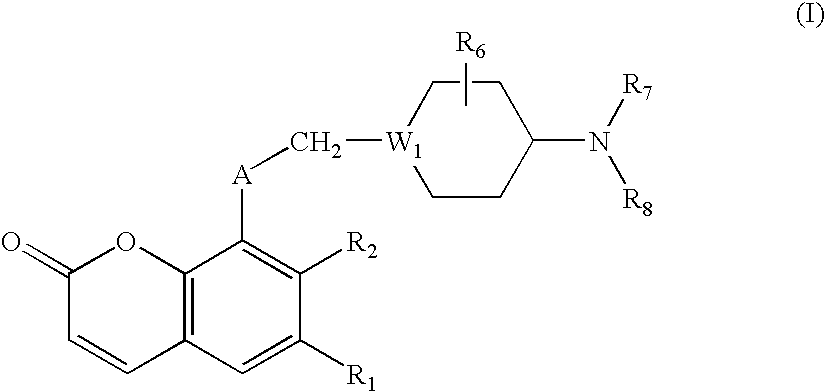
The present invention is directed to substituted 4H-chromenes, 2H-chromenes, chromans and analogs thereof, represented by the general Formula (I) wherein R5, A, B, X, Y, Z and dotted lines are defined herein. The present invention also relates to the discovery that compounds having Formula (I) are activators of caspases and inducers of apoptosis. Therefore, the activators of caspases and inducers of apoptosis of this invention can be used to induce cell death in a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.
Owner:CYTOVIA INC +1
2h-chromene compound and derivative thereof
[Object] Provided is a compound which has an excellent S1P1 agonist action, and is useful particularly as an active ingredient for an agent for preventing and / or treating a disease induced by undesirable lymphocyte infiltration or a disease induced by abnormal proliferation or accumulation of cells.[Means for Solution] According to the present invention, a 2H-chromene compound or a derivative thereof which has an excellent S1P1 agonist action, and is useful particularly as an active ingredient of an agent for preventing and / or treating a disease induced by undesirable lymphocyte infiltration or a disease induced by abnormal proliferation or accumulation of cells can be provided. The 2H-chromene compound and a derivative thereof which are the compounds of the present invention have an S1P1 agonist action, and can be used particularly for prevention and / or treatment of a disease induced by undesirable lymphocyte infiltration or a disease induced by abnormal proliferation or accumulation of cells.
Owner:ASTELLAS PHARMA INC
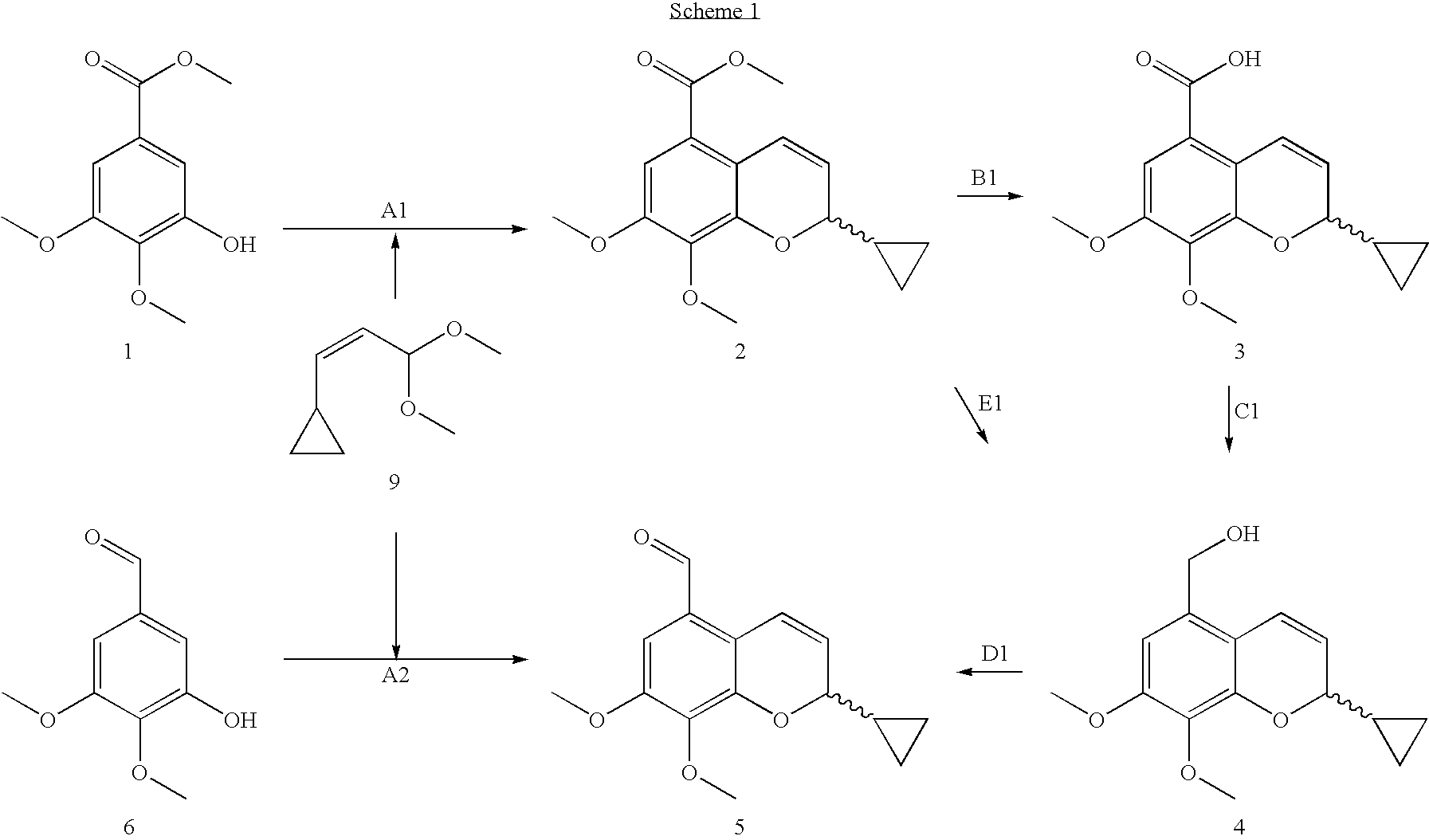
3-nitro-8-ethyoxyl-2H-chromene compound and preparation method and application thereof
InactiveCN102584768ANovel structureInhibit phosphorylationOrganic active ingredientsOrganic chemistryBenzopyran2H-chromene
The invention belongs to the technical field of medicine, and discloses a 3-nitro-8-ethyoxyl-2H-chromene compound (formula III) and a preparation method thereof. The invention further discloses an application of the compound to preparation of an antitumor medicament. In the formula III, R is H or Br.
Owner:SHANDONG UNIV
Novel Process For The Preparation Of 2H-Chromenes
Owner:ARPIDA AG
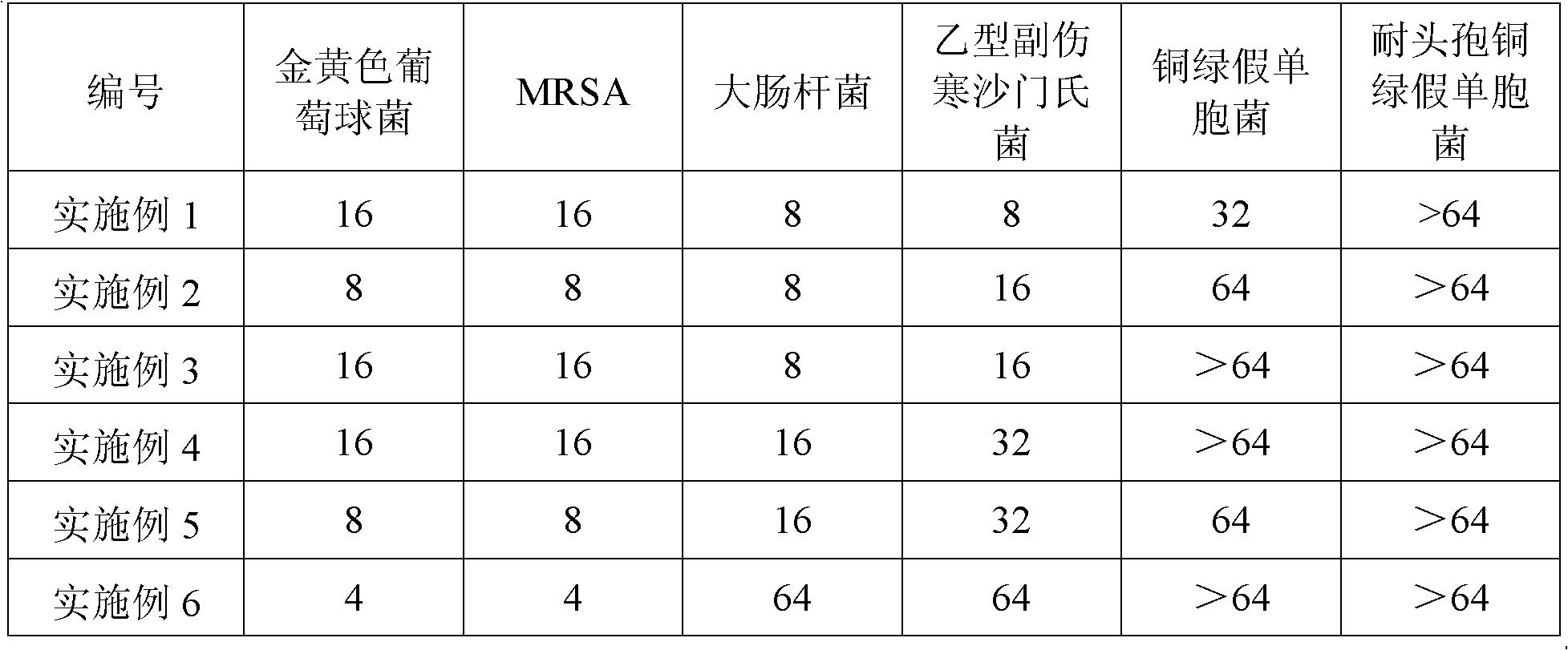
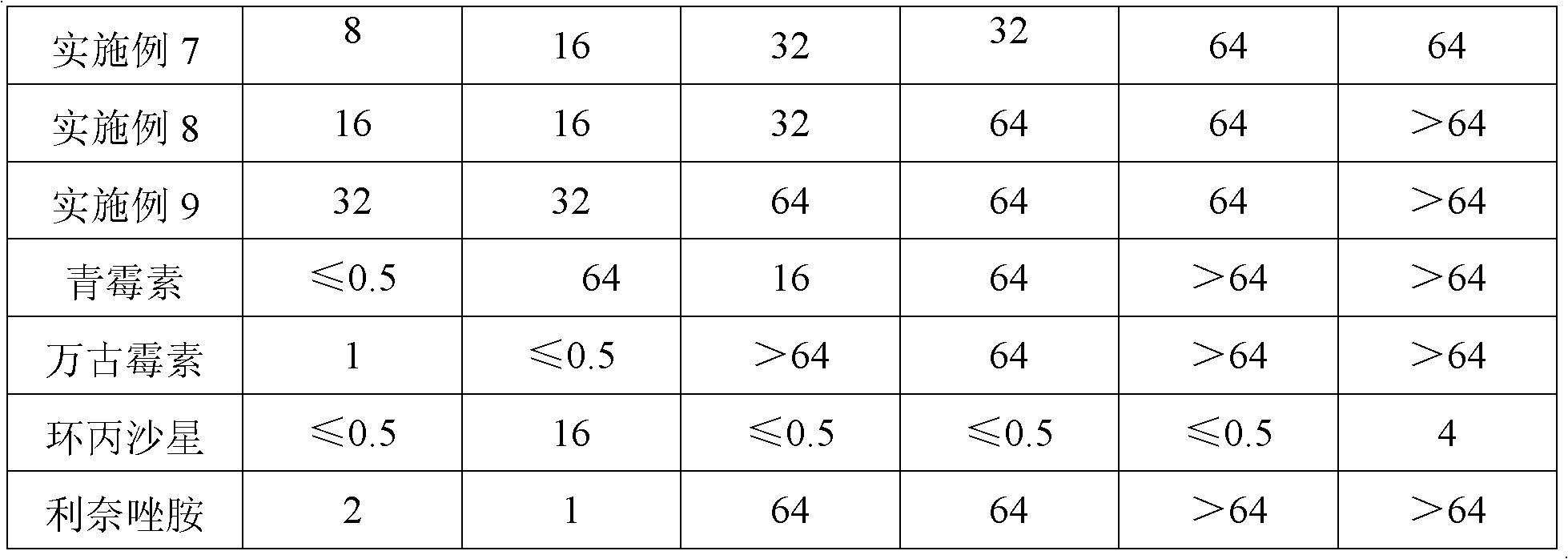
3-nitro-2h-chromene compounds with antibacterial activity and preparation method and application thereof
InactiveCN102153533ANew structureAvoid the conundrum of easy aggregationOrganic active ingredientsOrganic chemistrySalicylaldehydeAntibacterial activity
The invention discloses 3-nitro-2H-chromene compounds, a preparation method and application thereof. The 3-nitro-2H-chromene compounds have a novel antimicrobial parent nucleus structure, are prepared by reacting salicylaldehyde or substituted salicylaldehyde, n-Bu2NH, phthalic anhydride and 2-nitroethanol, and can be used for preparing medicaments for treating infectious diseases, particularly can be used for preparing medicaments for treating infectious diseases caused by multidrug-resistant bacteria.
Owner:SUN YAT SEN UNIV
Anti-estrogenic compounds
InactiveUS20160311805A1Reduce riskAvoid problemsOrganic chemistryAntineoplastic agentsAnti estrogenicEstrogen
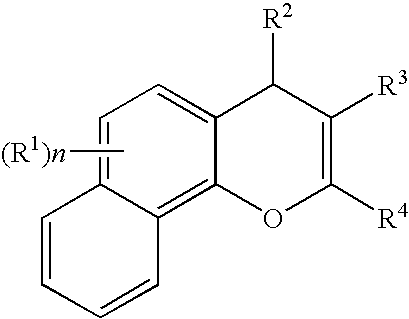
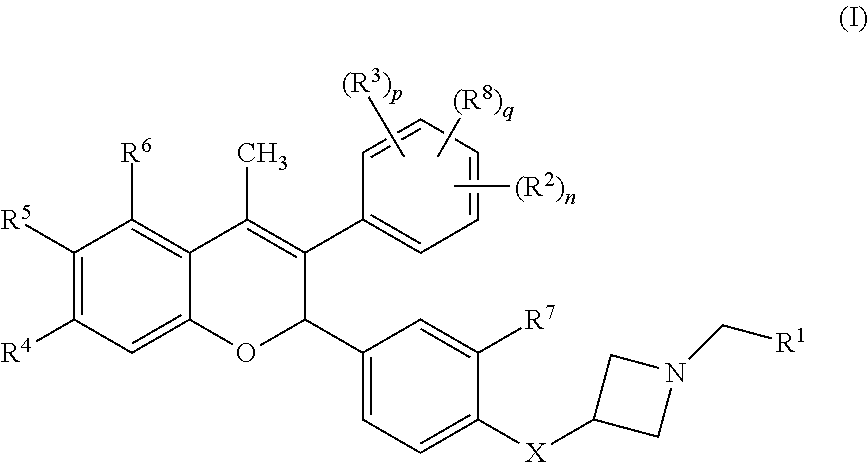
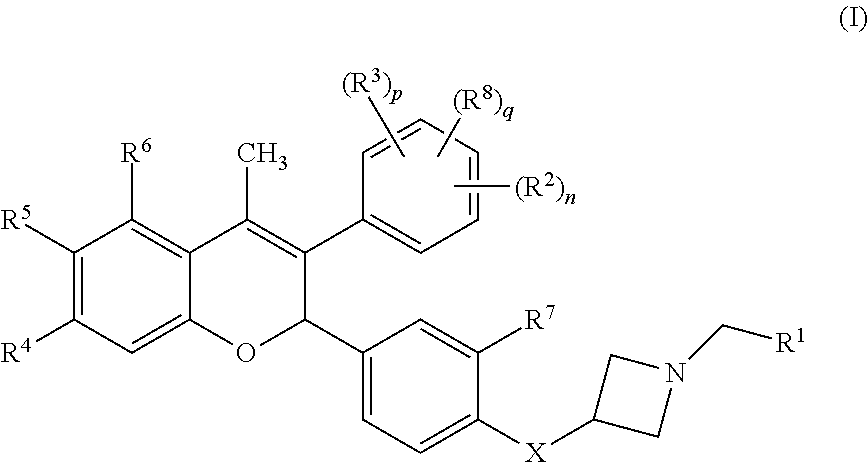
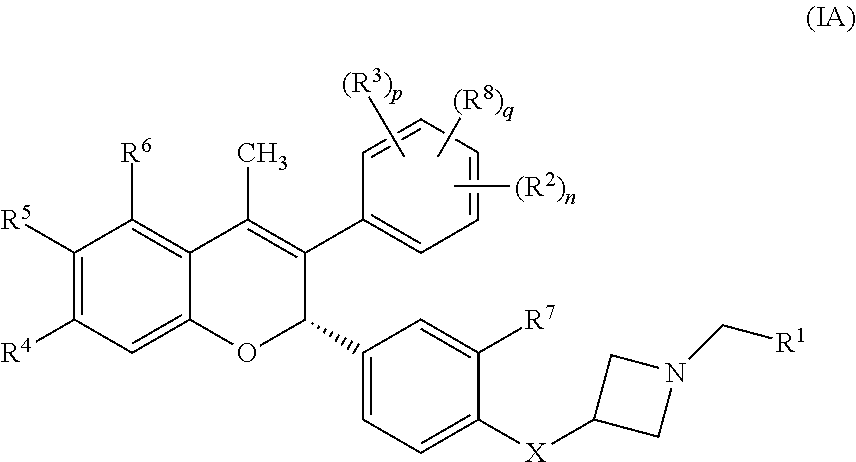
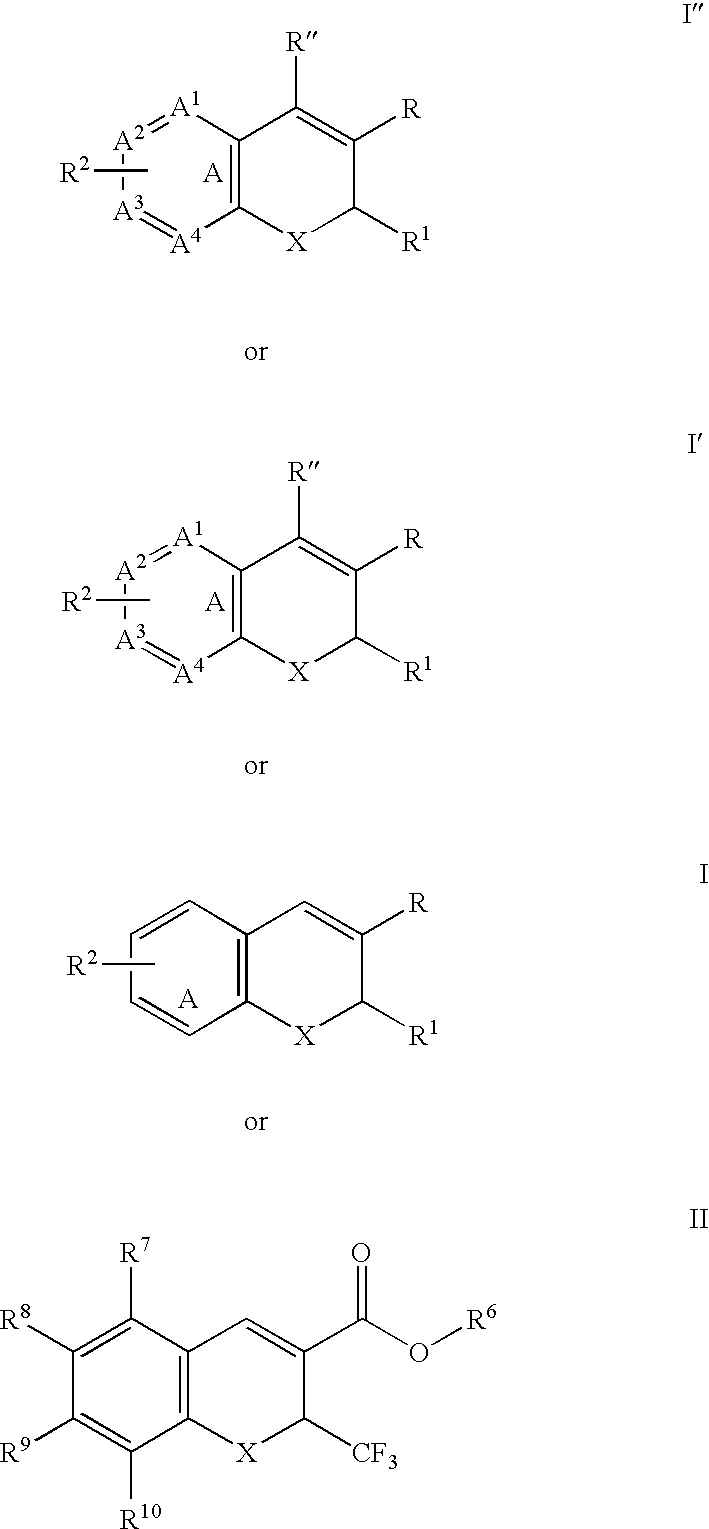
The present disclosure provides a compound of Formula I:or a pharmaceutically acceptable salt wherein X, R1-R8, Y1-Y5, m, n, p, and q are defined herein. The novel 2H-chromene compounds are useful for the modulation of disorders mediated by estrogen, and other disorders, as described herein. The present invention also relates to pharmaceutical compositions containing the compounds and to methods of using the compounds and compositions.
Owner:PFIZER INC
Photoracamization method
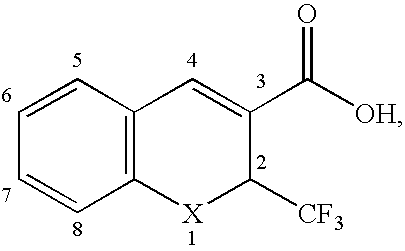
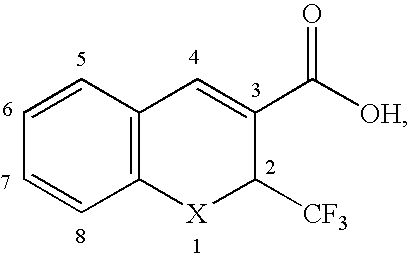
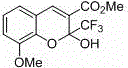
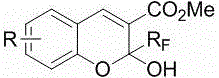
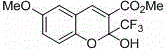
This invention relates to a method for photoracemizing enantiomers of a substituted 2-trifluoromethyl-2H-chromene-3-carboxylic acid or ester, a substituted 2-trifluoromethyl-1,2-dihydro-quinoline-3-carboxylic acid or ester, a substituted 2-trifluoromethyl-2H-thiochromene-3-carboxylic acid or ester, or a pharmaceutically acceptable salt of the acids or esters, using a high intensity UV light source.
Owner:PHARMACIA & UPJOHN CO
Method for preparing tolterodine and tartrate thereof
ActiveCN101445462AShort stepsLow costOrganic compound preparationOrganic chemistry methodsKetoneSolvent
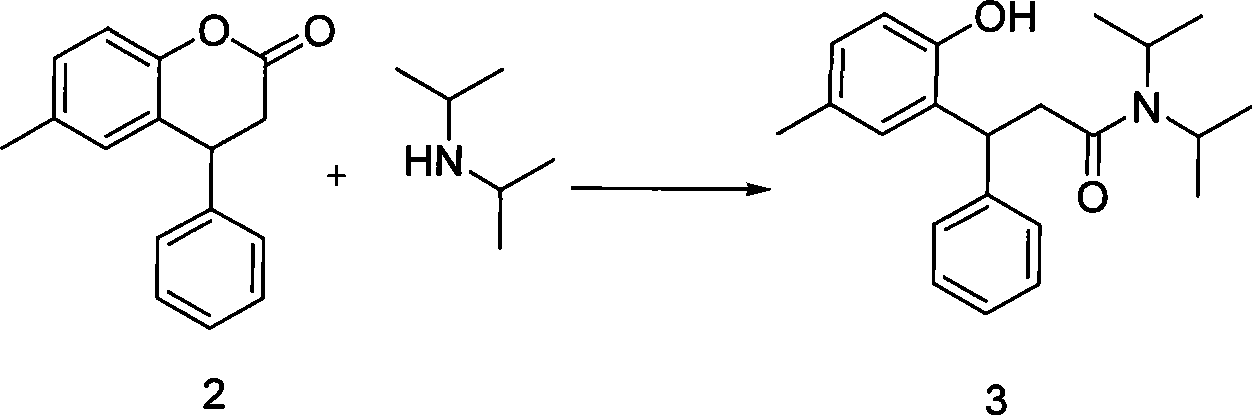
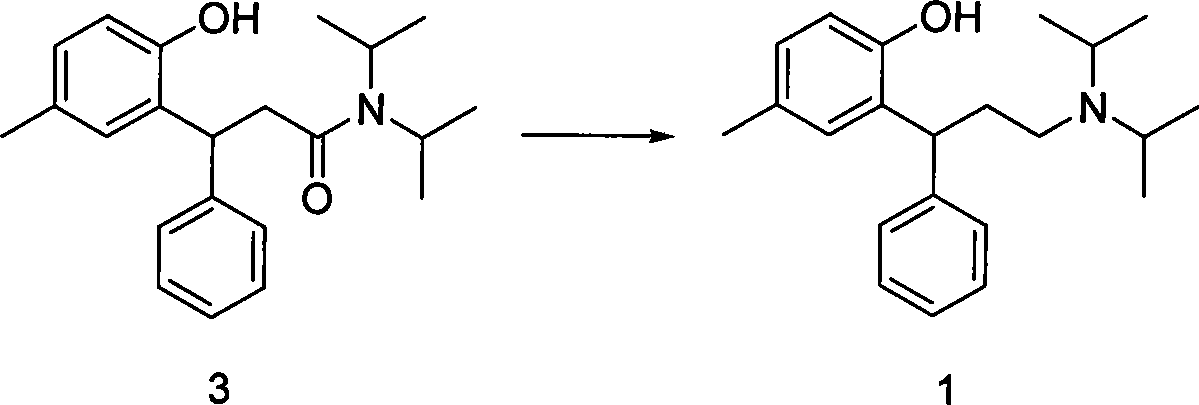
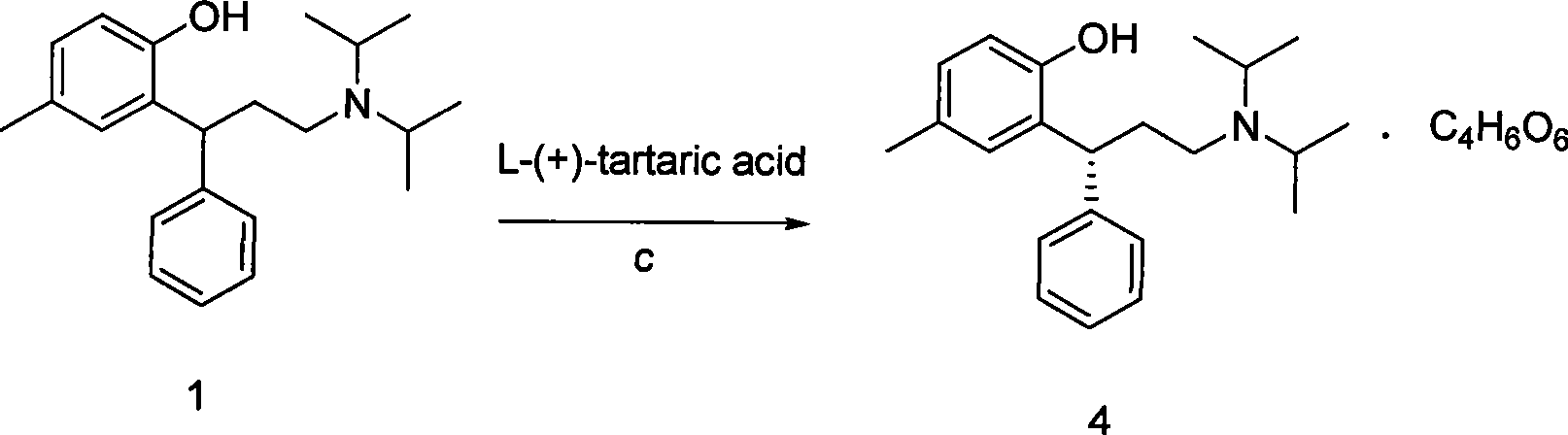
The invention relates to a method for preparing tolterodine and tartrate thereof, which comprises the steps: A) diisopropylamine and compound 2 (3, 4- dihydro-6- methyl-4-phenyl-2H-chromene-2- ketone can be activated and react, and then are decompressed and concentrated after being quenched, acidulated and extracted by organic solvent; after that, the mixture is added with crystallization solvent to be crystallized, and compound 3 is prepared; B) the compound 3 is deacidized by reducing agent, and then is quenched, separated and purified to obtain compound 1 tolterodine free alkali; C) the compound 1 tolterodine free alkali is dissolved by L-(+) tartaric acid, and compound 4 tolterodine tartrate is prepared. The method has short steps, low cost, high yield coefficient, easy operation and simple post treatment, and is stable in the quality of the prepared products and convenient for commercial process.
Owner:2Y CHEM
Fluorescence dye tagging scheme for mercury quantification and speciation
ActiveUS20130040393A1Large extinction coefficientHigh fluorescence quantum yieldOrganic chemistryTesting metalsFluorophoreHydroxy group
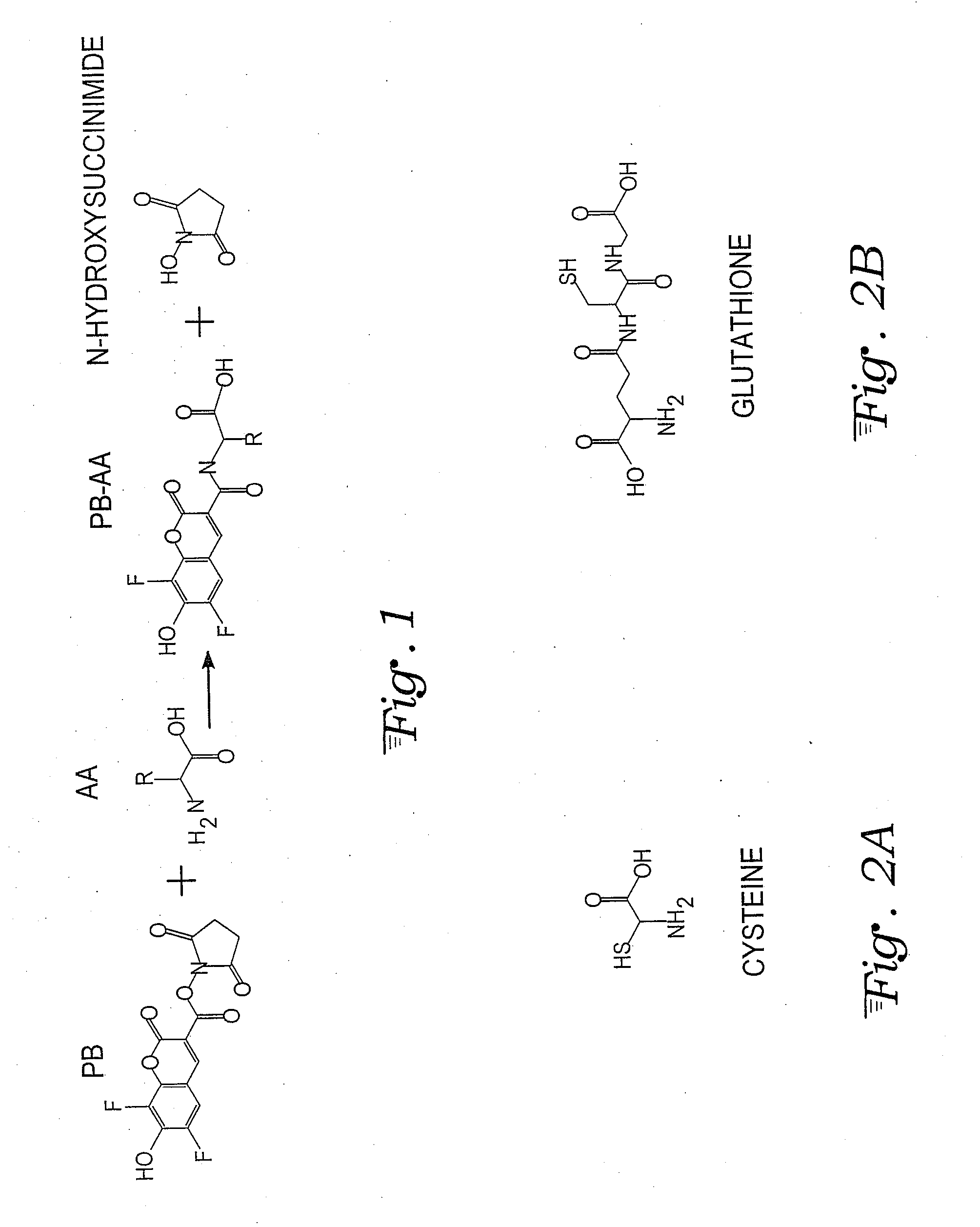
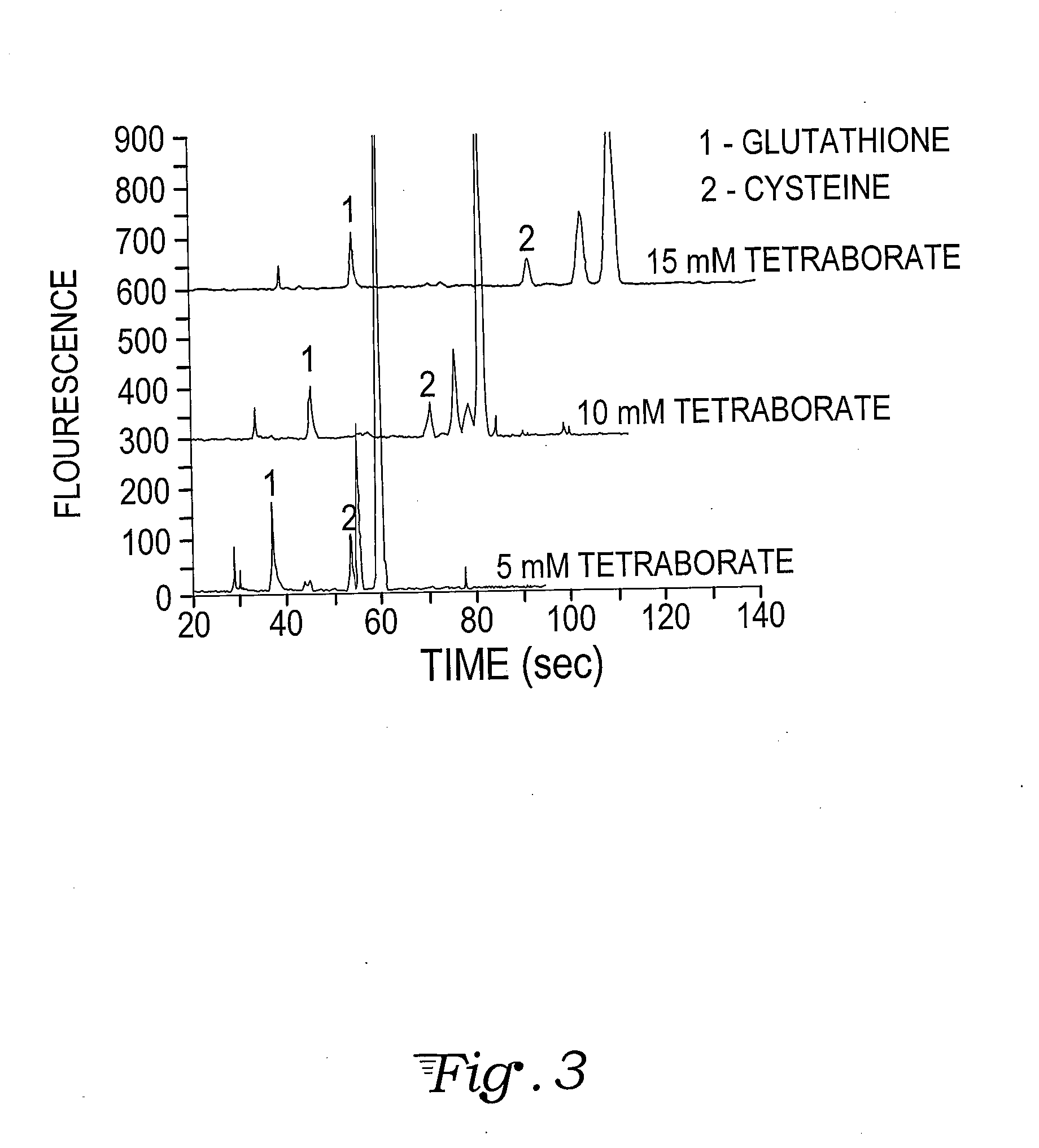
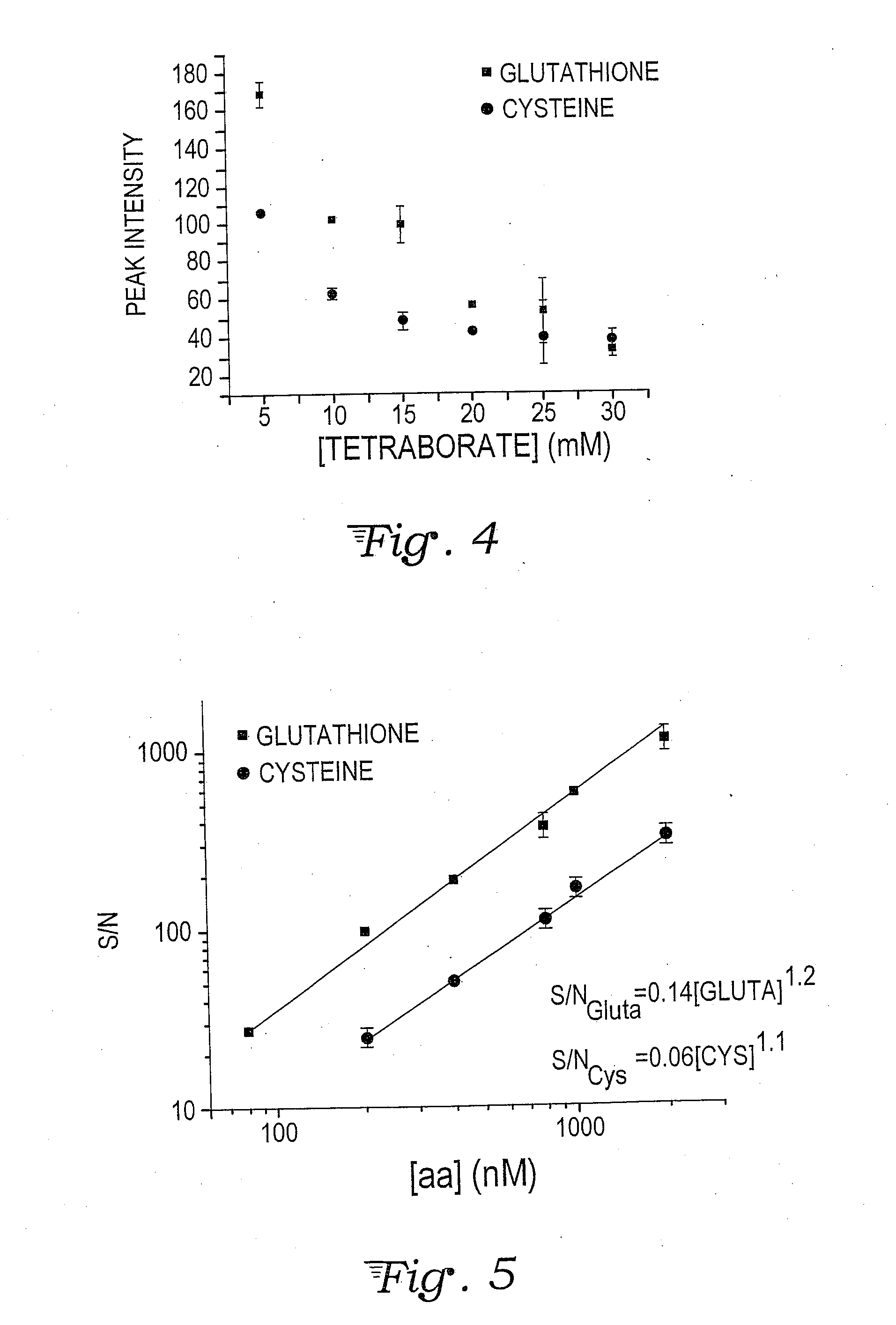
A fluorescent dye or fluorophore capable of forming complexes with mercury comprises 6,8-difluoro-7-hydroxy-2-oxo-2H-chromene-3-carboxylate amide, wherein the amide is formed by reacting the succinimidyl ester (Pacific Blue™) with an amino acid containing a thiol group, such as cysteine or glutathione. Mercury complexes of the fluorophore fluoresce when excited by a UV or violet laser diode, and the detected intensity can be calibrated to quantify the concentration of mercury in a sample reacted with the fluorophore.
Owner:ABB RES LTD
Two-photon fluorescent probe for detecting Cys as well as preparation method and application thereof
InactiveCN108484555ARealize identificationHigh fluorescence intensityOrganic chemistryFluorescence/phosphorescencePhototoxicityStructural formula
The invention provides a two-photon fluorescent probe for detecting Cys with the chemical name of 7-(diethylamino)-2-oxo-2H-chromene-3-acrylate, and the structural formula as shown in the description.The probe provided by the invention has small background interference, small scattering interference and high sensitivity and selectivity, can avoid photobleaching and phototoxicity, and can penetrate into tissue for deep imaging, and has broad application prospects in the field of biomolecule detection.
Owner:UNIV OF JINAN
Preparing method of (E)-N'-arylmethylene-4-(coumarin-3-yl)thiazole-2-hydrazide compound and its application
InactiveCN104817552AEnhanced inhibitory effectHigh activityAntibacterial agentsOrganic chemistryAcetic acidThiazole
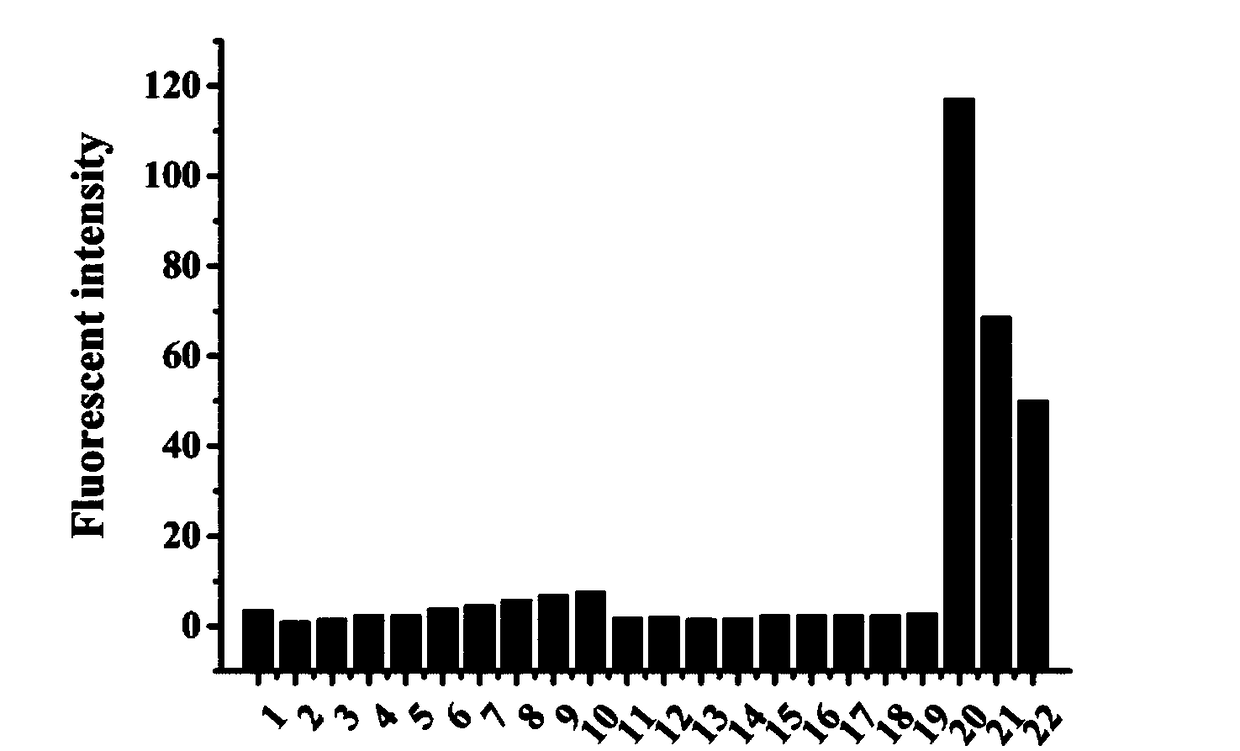
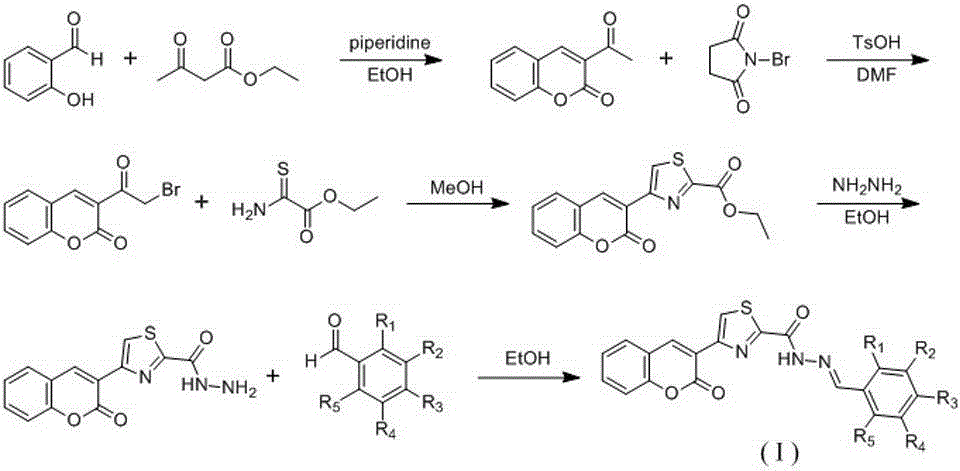
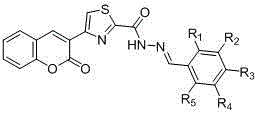
The invention discloses a preparing method of (E)-N'-arylmethylene-4-(coumarin-3-yl)thiazole-2-hydrazide compound and its application. A formula (I) of the compound is shown as below. The preparing method includes: making salicylic aldehyde and ethyl acetoacetate into 3-acetylindole-2H-chromene-2-one, allowing bromization, cyclization and hydrazinolysis to obtain 4-(2-oxo-2H-chromene-3-yl)thiazole-2-hydrazine, and allowing the 4-(2-oxo-2H-chromene-3-yl)thiazole-2-hydrazine to react with substituted benzaldehydes to obtain the target compound. The compound can serve as a raw material for antiseptic medicine; the preparing method has the advantages that the raw materials are simple and easy to obtain and operating is convenient.
Owner:JISHOU UNIVERSITY
Antibacterial Agents
Owner:GLAXO GROUP LTD
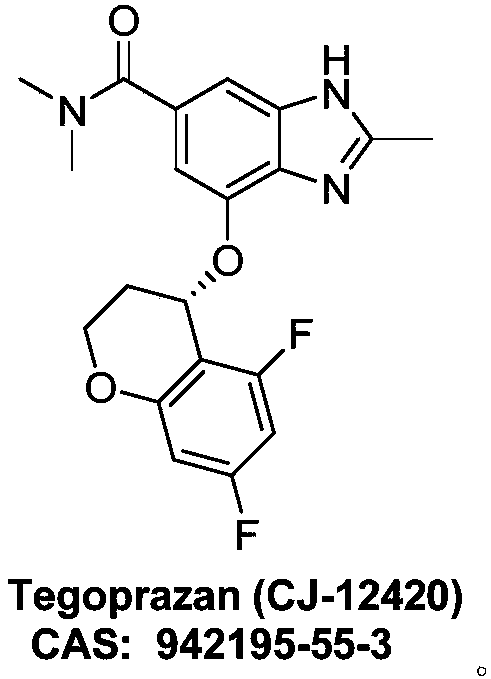
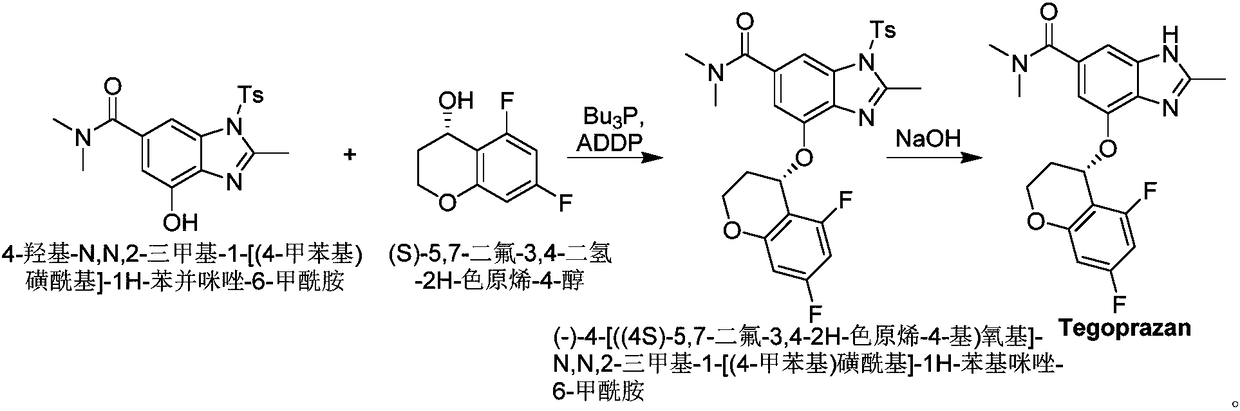
Method for synthetizing Tegoprazan chiral alcohol
ActiveCN109320485AThe synthesis method is simpleConvenient sourceOrganic chemistry methodsBenzopyranAlcohol
Owner:WISDOM PHARM CO LTD
2H-chromene compound and derivative thereof
Provided is a compound which has an excellent S1P1 agonist action, and is useful particularly as an active ingredient for an agent for preventing and / or treating a disease induced by undesirable lymphocyte infiltration or a disease induced by abnormal proliferation or accumulation of cells. According to the present invention, a 2H-chromene compound or a derivative thereof which has an excellent S1P1 agonist action, and is useful particularly as an active ingredient of an agent for preventing and / or treating a disease induced by undesirable lymphocyte infiltration or a disease induced by abnormal proliferation or accumulation of cells can be provided. The 2H-chromene compound and a derivative thereof which are the compounds of the present invention have an S1P1 agonist action, and can be used particularly for prevention and / or treatment of a disease induced by undesirable lymphocyte infiltration or a disease induced by abnormal proliferation or accumulation of cells.
Owner:ASTELLAS PHARMA INC
Racemization method
This invention relates to a method for racemizing enantiomers of a substituted 2-trifluoromethyl-2H-chromene-3-carboxylic acid or ester, a substituted 2-trifluoromethyl-1,2-dihydro-quinoline-3-carboxylic acid or ester, a substituted 2-trifluoromethyl-2H-thiochromene-3-carboxylic acid or ester, or a pharmaceutically acceptable salt of the acids or esters, using secondary amines, and optionally hydroxides, alkoxides, or sulfites at reaction mixture temperatures of from about 30° C. (i.e., above room temperature) to less than 300° C.
Owner:PHARMACIA & UPJOHN CO
Photochromic 2h-chr0menes annulated at c5-c6 and their methods of preparation
InactiveCN102131793AImproves Fade KineticsHigh reaction yieldOrganic chemistryOptical partsPhotochromismTemperature sensitive
Described herein are C5-C6 annulated naphthopyrans that possess at least one electron-withdrawing group. The compounds possess desirable properties such as increased fading kinetics. Also described herein are new methods for synthesizing 2H- chromenes annulated at C5-C6. The methods involve less stringent reaction conditions as well as provide increased reaction yields. The methods permit the synthesis of a wide variety of substituted naphthopyrans that can be temperature sensitive and were not possibly synthesized with previous synthetic routes.
Owner:CORNING INC
Preparation method of 4-tosylester-2H-chromene
InactiveCN109232501AMild reaction conditionsSimple and fast operationOrganic chemistryChemical synthesisP-Toluenesulfonic acid
The invention discloses a preparation method of 4-tosylester-2H-chromene, wherein ortho propargyl alcohol phenol is used as a raw material, an organic solvent is added to dissolve fully at room temperature, and then p-toluenesulfonic acid (p-TsOH) is added and reacted at 60 DEG C for 1 to 2 hours. After the reaction is completed, the reaction product is separated and purified by column chromatography on silica gel to obtain the 4-tosylester-2H-chromene. The invention has the characteristics of simple and easily accessible raw materials, mild reaction conditions, simple operation, short reaction time and less pollution and the like, and is a better chemical synthesis method with popularization and application prospects.
Owner:JIANGXI SCI & TECH NORMAL UNIV
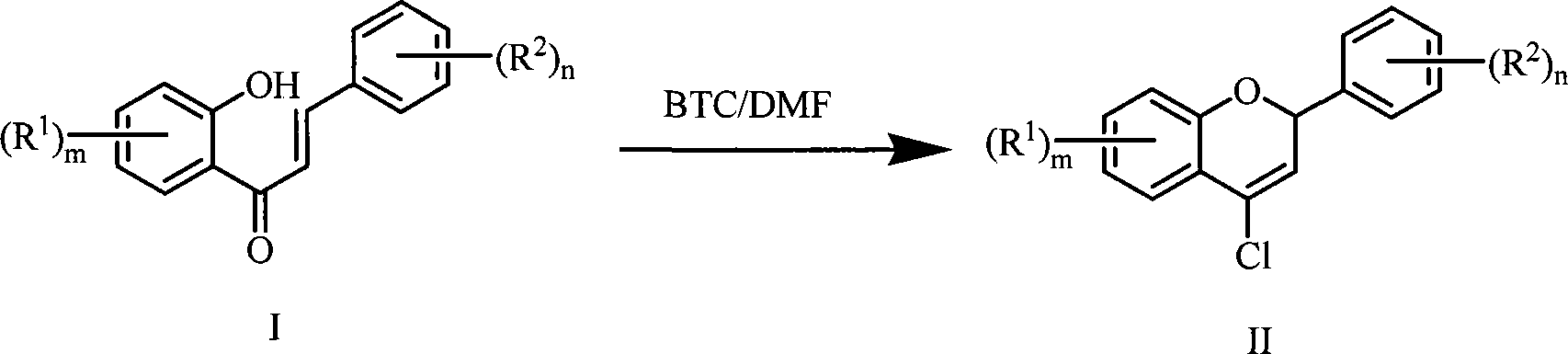
Method for preparing 4-chlorine-2H-chromene derivative
InactiveCN101225079AReduce dosageWiden the optionsOrganic chemistryChemical synthesisN dimethylformamide
The invention discloses a preparation method for 4-Cl-2H-chromene, which is characterized in that: as raw material, bis-(trichloromethyl) carbonate (BTC) and N, N-dimethylformamide (DMF) are reacted fully in organic solvent at the temperature of 0 to 5 DEG C; then substituted o-hydroxy chalcone compound as shown in the formula (I) is added for reaction for 0.5 to 2 hours at the temperature of 0 to 5 DEG C before heated to 60 to 90 DEG C for full reaction; 4-Cl-2H-chromene derivative as shown in the formula (II) can be get through separating and purifying reaction products after the reaction is ended. The preparation method for 4-Cl-2H-chromene has the advantages of easy availability for raw material, mild reaction condition, convenient operation, high yield and chemical synthetic method with better popularization and application prospect. The preparation method for 4-Cl-2H-chromene abolishes phosphate pollution source and greatly lowers the content of N, N-dimethylformamide.
Owner:ZHEJIANG UNIV OF TECH
Antibacterial agents
Owner:GLAXO GRP LTD
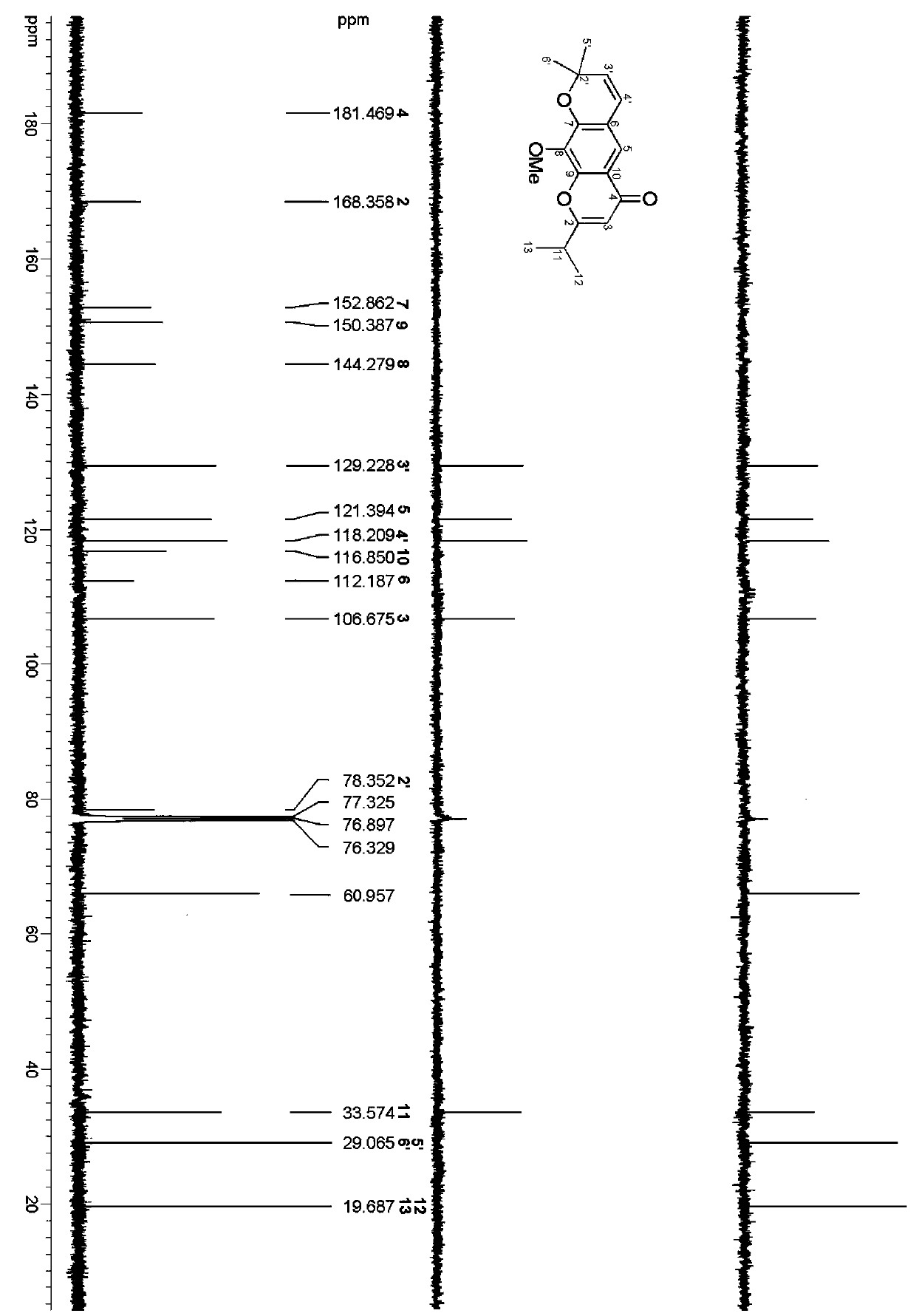
Chromone derivative, preparation method and application thereof
ActiveCN109535169ASimple structureHigh activityBiocideOrganic chemistryChromatographic separationTobacco mosaic virus
The invention discloses a chromone derivative, a preparation method and application thereof. The chromone derivative is separated from a cassia leschenaultiana whole plant, the molecular formula is C18H20O4, and the compound is named as: 6-(2, 2-dimethyl-2H-chromene)-2-isopropyl-8-methoxy-4H-chromen-4-one. The preparation method of the chromone derivative includes: taking a cassia leschenaultianawhole plant as the raw material, and conducting crushing, extraction, silica gel column chromatography and high pressure liquid chromatography separation steps. Biological activity test indicates thatthe compound provided by the invention has a good inhibiting effect on tobacco mosaic virus. The compound provided by the invention is easily separable, has good activity, and can be used as a leading compound against tobacco mosaic virus.
Owner:CHINA TOBACCO YUNNAN IND
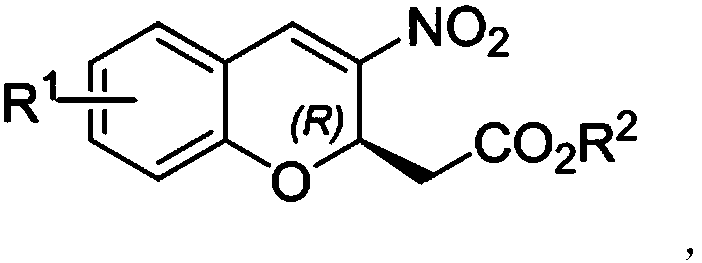
R-2-substituted-3-nitro-2H-chromene derivative with antibacterial activity and preparation method and application thereof
ActiveCN108409696AHigh optical activityImprove the bactericidal effectAntibacterial agentsOrganic chemistry methodsSalicylaldehydePolarimeter
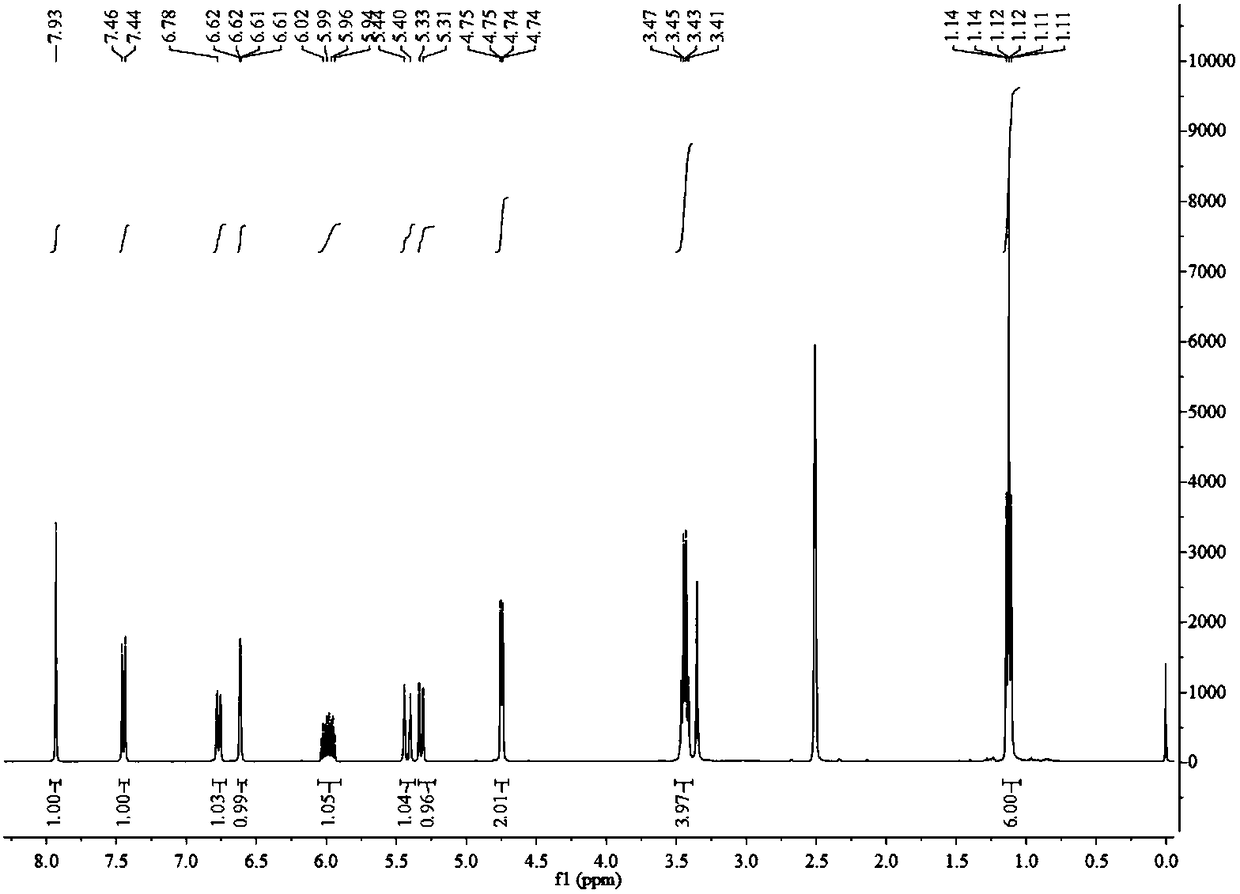
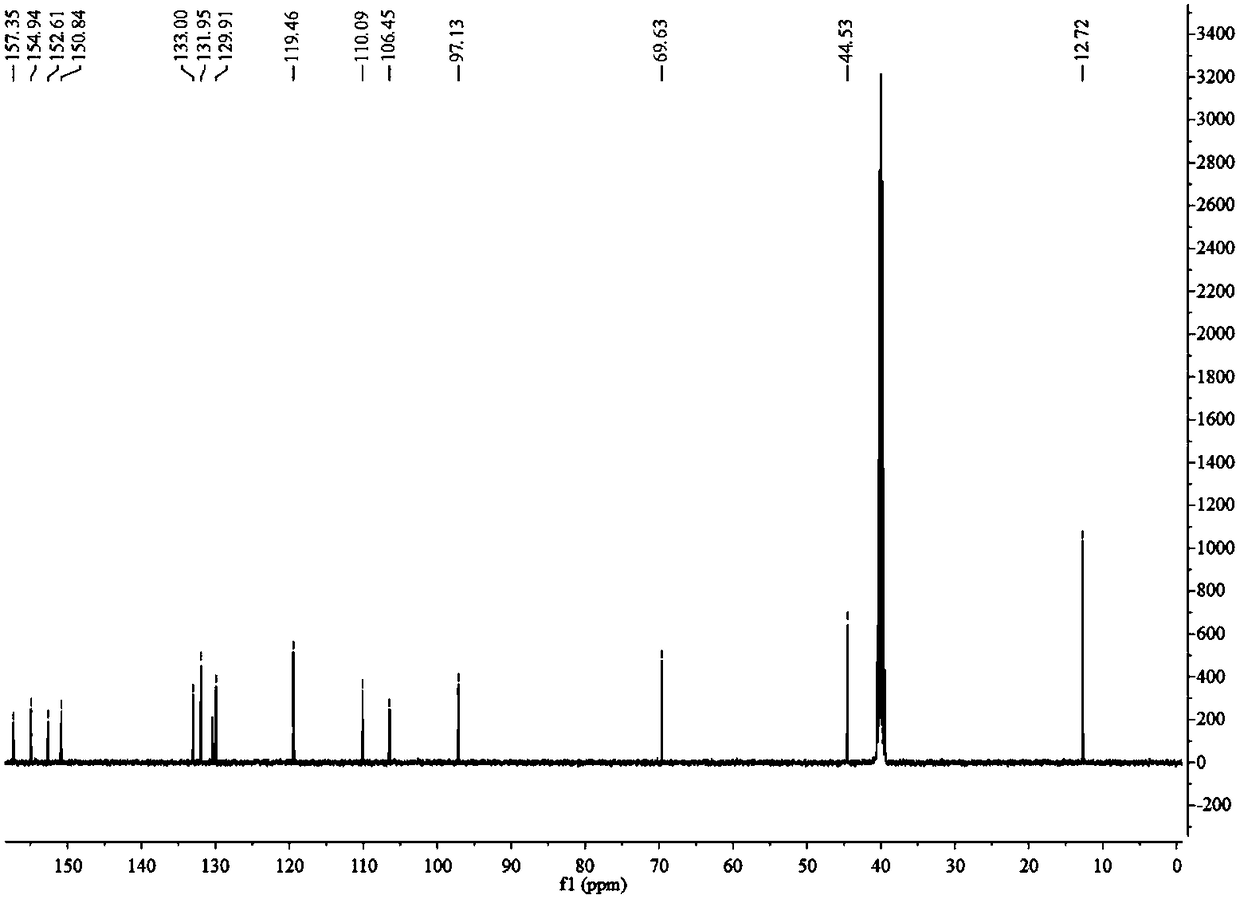
The invention discloses an R-2-substituted-3-nitro-2H-chromene derivative with antibacterial activity and a preparation method and application thereof. The R-2-substituted-3-nitro-2H-chromene derivative belongs to chiral compounds of R configuration. The preparation method is easy. The R-2-substituted-3-nitro-2H-chromene derivative is prepared through organic catalysis of a salicylaldehyde derivative and nitromethane in a 'one-pot method'. Compared with a raceme, the effective single configuration of product obtained through asymmetrical synthesis has higher antibacterial activity, eliminatingimpact of another ineffective configuration. The R-2-substituted-3-nitro-2H-chromene derivative is represented by using the means of 1H NMR, 13C NMR, HRMS, a polarimeter and the like. Optical pure R-2-substituted-3-nitro-2H-chromene derivative has high antibacterial activity, and can be used for preparing chiral antibacterial agents.
Owner:SOUTHWEST UNIV
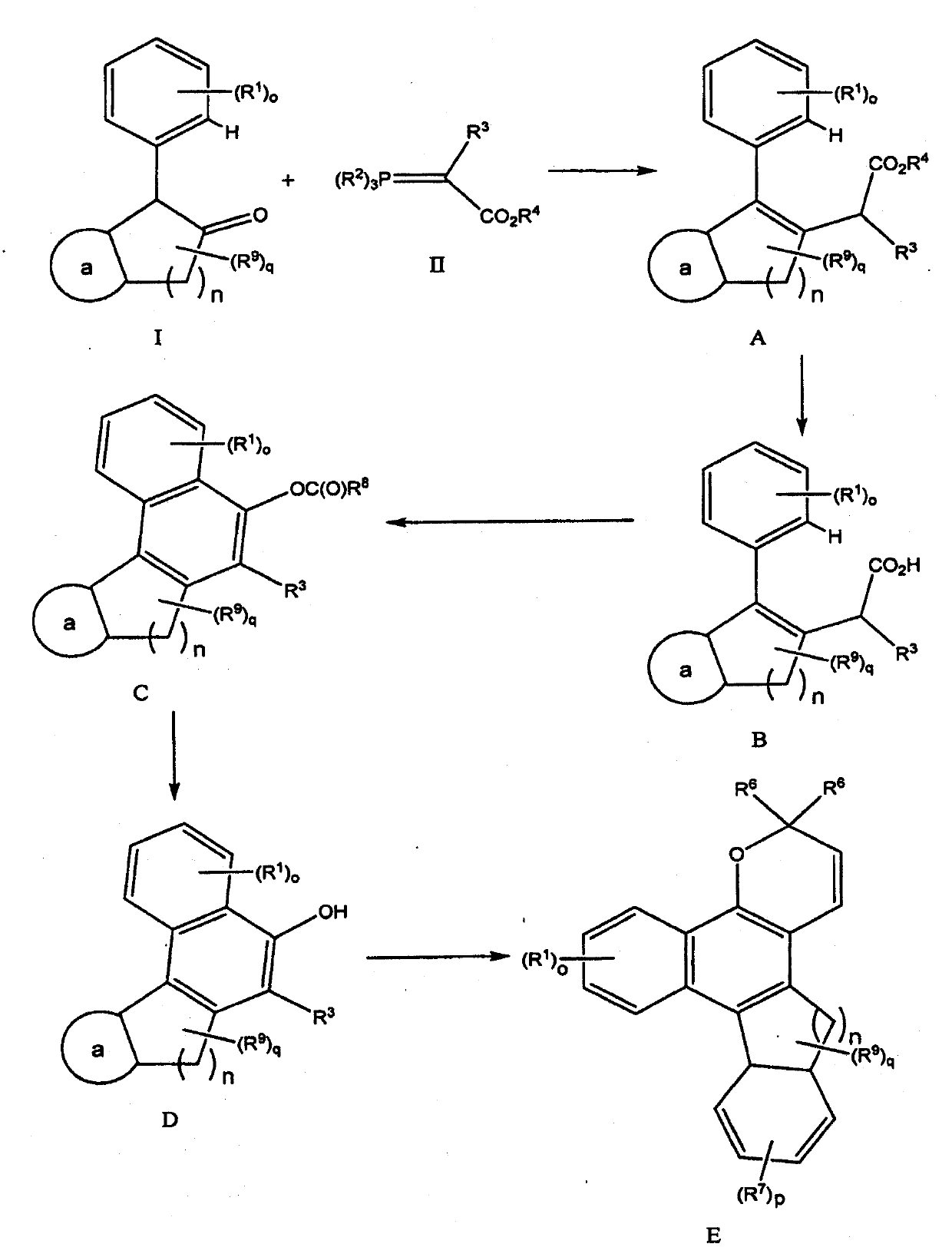
Novel Processes For The Preparation Of A 2H-Chromene
The present invention relates to novel processes for the preparation of the compound of formula I (Iclaprim), related to dihydrofolate reductase inhibitorsand to valuable intermediates of these processes.
Owner:ARPIDA AG
Substituted 4H-chromens, 2H-chromenes, chromans and analogs as activators of caspases and inducers of apoptosis and the use thereof
The present invention is directed to substituted 4H-chromenes, 2H-chromenes, chromans and analogs thereof, represented by the general Formula (I) wherein R5, A, B, X, Y, Z and dotted lines are defined herein. The present invention also relates to the discovery that compounds having Formula (I) are activators of caspases and inducers of apoptosis. Therefore, the activators of caspases and inducers of apoptosis of this invention can be used to induce cell death in a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.
Owner:CYTOVIA INC +1
2H-chromene derivatives as analgesic agents
The invention relates to the use of the compound of general formula 1 or its spatial isomersas an analgesic drug. The compounds have high activity, low toxicity, may be used in medicine.
Owner:OBSHESTVO S OGRANICHENNOI OTVETSVENNOSTJU LEOFORS
Process for the preparation of 2H-chromenes
Owner:ARPIDA AG
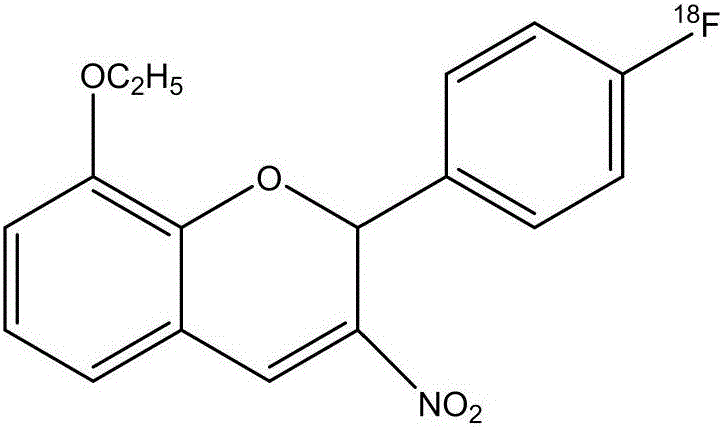
<18>F-labeled PI3K/Akt signal path inhibitor S14161, and preparation method and application thereof
ActiveCN106543131AC-F bond strengthThere will be no off-label phenomenonRadioactive preparation carriersIsotope introduction to organic compoundsChemical synthesisAkt signalling
The invention discloses a <18>F-labeled-8-ethoxy-2-(4-fluorophenyl)-3-nitro-2H-chromene (S14161) and a preparation method thereof. The preparation method has the following advantages: preparation raw materials are cheap and are easy to obtain, the synthesis process is simple, reaction conditions are mild, and the radiochemical synthesis time is short; the above product is simple and convenient to purify and separate, the radiochemical purity of the product exceeds 98%, the radiochemical yield of the product is high, the uncorrected yield reaches up to 92%, and the radioactivity of the product is high; and the product is useful in the inspection and diagnosis of tumors and the radiation therapy of the tumors. The structural formula of the <18>F-labeled-8-ethoxy-2-(4-fluorophenyl)-3-nitro-2H-chromene is shown in the description.
Owner:SHANDONG UNIV QILU HOSPITAL
2h-chromene derivatives as analgesic agents
The invention relates to the use of the compound of general formula 1 or its spatial isomersas an analgesic drug. The compounds have high activity, low toxicity, may be used in medicine.
Owner:OBSHESTVO S OGRANICHENNOI OTVETSVENNOSTJU LEOFORS
2H-chromene derivative containing perfluoroalkyl group, and synthetic method thereof
The present invention relates to a kind of containing perfluoroalkyl 2 H Chromene derivatives and synthetic methods thereof, the structure of the compound is: Wherein, R is H, methoxy, bromo, methyl or nitro; RF is trifluoromethyl or pentafluoroethyl. The method of the present invention is synthesized by two-component one-pot method, compared with the method of original report, this method uses L -Proline as a green and mild organic catalyst to synthesize perfluoroalkyl-containing 2 H -Chromene derivatives have the advantages of high atom economy, environmental friendliness, easy operation and mild conditions. The reaction uses anhydrous methanol as a solvent, which has little environmental pollution. High regioselectivity and good yield. Therefore, this method is the synthesis of perfluoroalkyl-containing 2 H - Efficient new approach to chromene derivatives.
Owner:SHANGHAI UNIV
Fungicide enhancers effective for treating plants infected with fungal pathogens
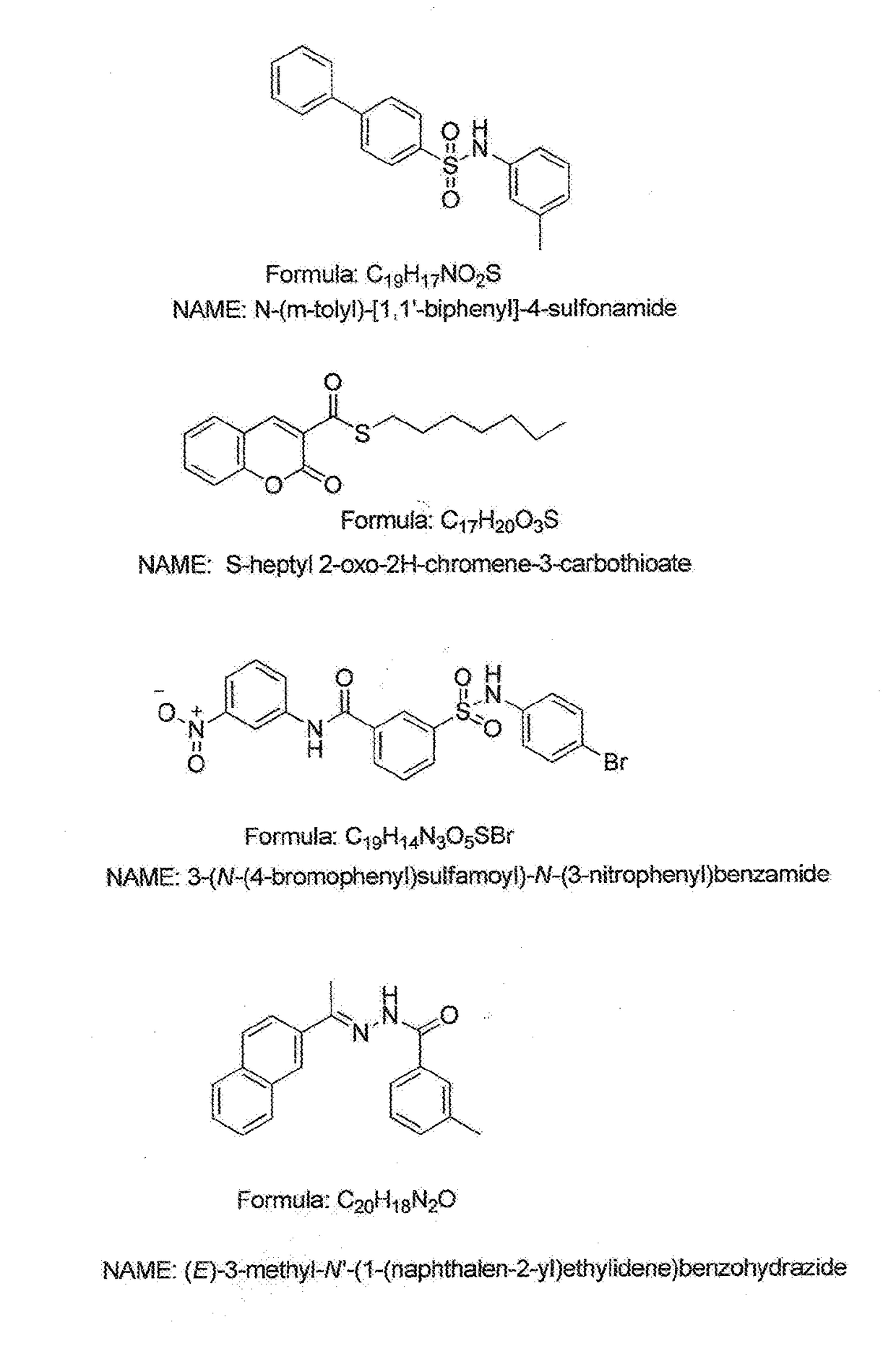
The present invention includes compositions and methods of for treating plants infected with fungal pathogens by contacting an infected plant or plant at risk of infection with a fungicidal composition comprising an fungicide selected from copper compound such as copper octanoate or copper hydroxide, or a triazole fungicide such as myclobutanil, propiconazole, tebuconazole or epoxiconazole, an enhancer selected from apyrase inhibitors, e.g., N-(m-toly1)-[1, 1′-biphenyl]-4-sulfonamide, S-heptyl 2-oxo-2H-chromene-3-carbothioate, 3-(N-(4-bromophenyl) sulfamoyl)-N-(3-nitrophenyl) benzamide, or (E)-3-methyl-N-(1-(naphthalen-2-yl) ethylidene) benzohydrazide and, optionally, a phytologically-acceptable inert carrier.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com