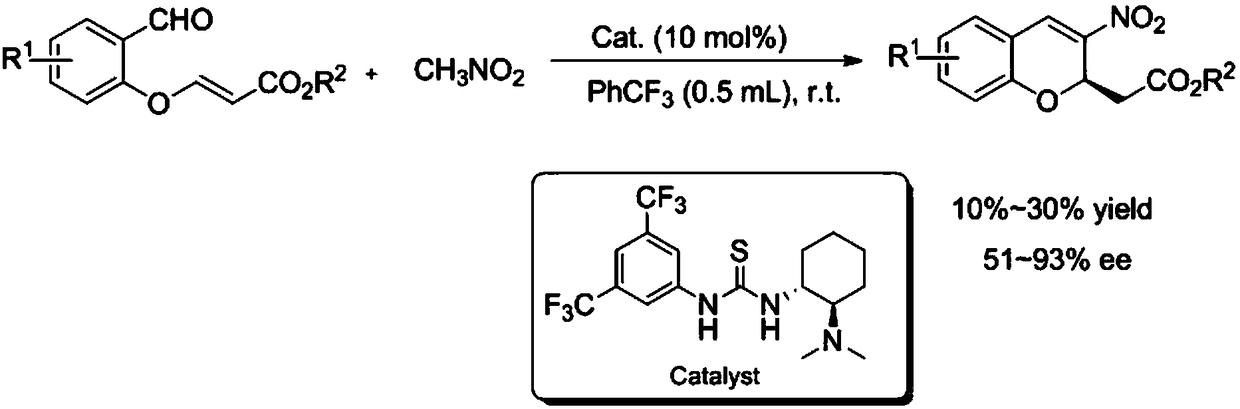
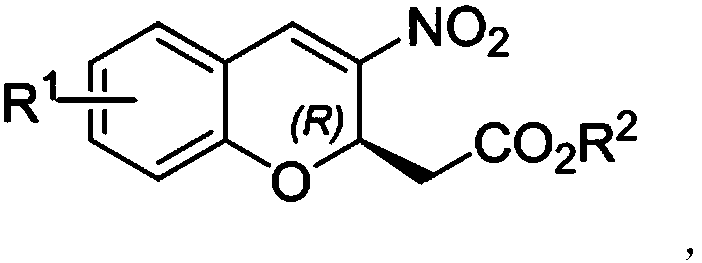
R-2-substituted-3-nitro-2H-chromene derivative with antibacterial activity and preparation method and application thereof
An R-2-, antibacterial activity technology, applied in organic chemical methods, antibacterial drugs, organic chemistry and other directions, can solve problems such as low recovery rate, achieve good bactericidal performance, good application value, and reduce the effect of dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Preparation of (R)-2-(3-nitro-2H-chromene) ethyl acetate I-1:
[0024] At room temperature, add 3-(2-formylphenoxy) ethyl acrylate II-1 (0.2mmol, 1.0equiv), nitromethane (1mmol, 5equiv) and catalyst (0.01mmol, 0.1equiv) to the sealed tube. ) And PhCF 3 (0.5mL). The reaction mixture was stirred at room temperature for 72 hours, and the reaction was checked by TLC. After the reaction, the crude product was purified by silica gel column chromatography and eluted with petroleum ether / ethyl acetate (20:1) to obtain 15.0 mg of yellow oily liquid with a yield of 27%. Use a mixed solution of petroleum ether / ethyl acetate = 10 / 1 as a developing agent, R f =0.23, (R)-2-(3-nitro-2H-chromene) ethyl acetate I-1 was prepared, and its chemical structure is as follows:
[0025]
[0026] 1 H NMR(400MHz, CDCl 3 ): δ (ppm) = 7.85 (s, 1H), 7.38 (td, J = 8.2, 1.6 Hz, 1H), 7.29 (dd, J = 7.6, 1.6 Hz, 1H), 7.04 (td, J = 7.5, 1.1Hz, 1H), 6.94 (d, J = 8.2 Hz, 1H), 6.04 (dd, J = 9.4, 3.6...
Embodiment 2
[0031] Example 2: Preparation of (R)-2-(6-methoxy-3-nitro-2H-chromene) ethyl acetate 1-2
[0032] At room temperature, add 3-(2-formyl-4-methoxyphenoxy) ethyl acrylate II-2 (0.2mmol, 1.0 equiv), nitromethane (1mmol, 5equiv) and catalyst to the sealed tube (0.01mmol, 0.1equiv) and PhCF 3 (0.5mL). The reaction mixture was stirred at room temperature for 72 hours, and the reaction was checked by TLC. After the reaction, the crude product was purified by silica gel column chromatography and eluted with petroleum ether / ethyl acetate (20:1) to obtain 31.5 mg of yellow oily liquid with a yield of 27%. Use a mixed solution of petroleum ether / ethyl acetate = 10 / 1 as a developing agent, R f = 0.3, compound I-2 is prepared, and its chemical structure is as follows:
[0033]
[0034] 1 H NMR(400MHz, CDCl 3 ):δ(ppm)=7.80(s,1H), 6.95(dd,J=8.9,2.9Hz,1H), 6.89-6.84 (m,1H), 6.79(d,J=2.9Hz,1H), 5.98 (dd,J=9.5,3.6Hz,1H),4.23-4.13(m,2H),3.79(s,3H), 2.83(dd,J=15.0,9.5Hz,1H),2.66(dd,J=15.0 , 3.6 Hz,...
Embodiment 3
[0039] Example 3: Preparation of (R)-2-(7-methoxy-3-nitro-2H-chromene) ethyl acetate I-3
[0040] At room temperature, add 3-(2-formyl-5-methoxyphenoxy) ethyl acrylate II-3 (0.2mmol, 1.0 equiv), nitromethane (1mmol, 5equiv) and catalyst to the sealed tube (0.01mmol, 0.1equiv) and PhCF 3 (0.5mL). The reaction mixture was stirred at room temperature for 72 hours, and the reaction was checked by TLC. After the reaction, the crude product was purified by silica gel column chromatography and eluted with petroleum ether / ethyl acetate (20:1) to obtain 14.3 mg of yellow oily liquid with a yield of 12%. Use a mixed solution of petroleum ether / ethyl acetate = 10 / 1 as a developing agent, R f =0.25, compound I-3 is prepared, and its chemical structure is as follows:
[0041]
[0042] 1 H NMR(400MHz, CDCl 3 ): δ (ppm) = 7.85 (s, 1H), 7.20 (d, J = 8.5 Hz, 1H), 6.60 (dd, J = 8.5, 2.4 Hz, 1H), 6.47 (d, J = 2.4 Hz, 1H ), 6.04 (dd, J = 9.5, 3.4 Hz, 1H), 4.20 (dtq, J = 10.8, 6.8, 3.7 Hz, 2H), 3.83...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com