Method for preparing tolterodine and tartrate thereof
A technology of tartrate and tolterodine, applied in the field of compounds, can solve the problems of increased production cost, high price of cinnamon chloride, difficult availability of raw materials, etc., and achieves the effects of easy operation, simple post-processing and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
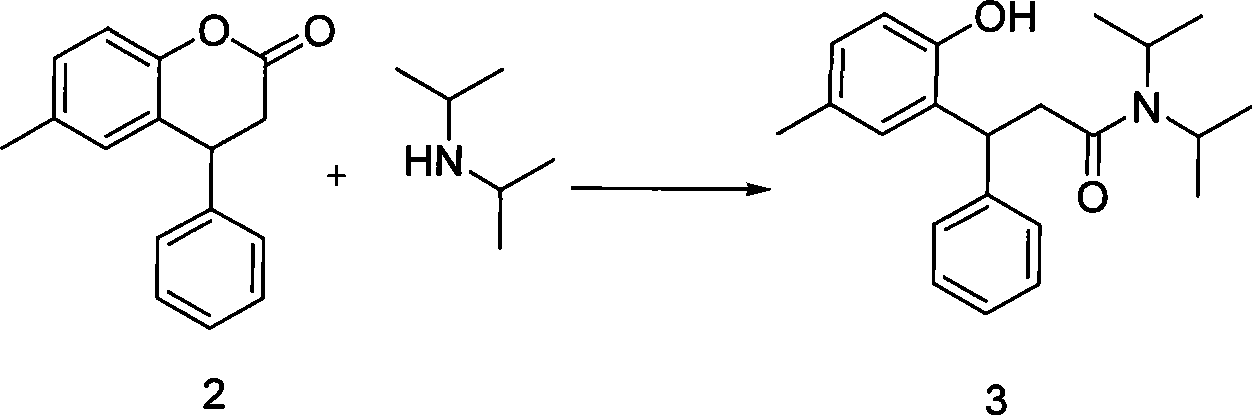
[0033] in N 2 Under protection, Mg scraps (1.0g, 0.042mol) were heated to 65°C in a 100ml three-necked flask. After half an hour, 30ml of tetrahydrofuran was added, and bromoethane (4.6g, 0.042mol) was diluted with 10ml of tetrahydrofuran (2 drops of bromine Ethane triggers the reaction), slowly drop into the reaction flask, and keep the reflux temperature (65°C). Diisopropylamine (6.4 g, 0.063 mol) was added after the Mg scraps completely disappeared, and refluxed for 1 hour, then a solution of compound 2 in THF (5 g, 0.021 mol dissolved in 10 ml THF) was added, and the temperature (65° C.) was kept at reflux overnight. After the reaction was complete, 10 ml of water was added to quench the reaction, and the pH value was adjusted to 3 with HCl, and 50 ml of ethyl acetate was added to separate the layers, the organic phase was washed with water (30 ml*3), and dried. The solvent was distilled off under reduced pressure, and the residue was crystallized by adding 10 volumes of ...
Embodiment 2
[0037] in N 2 Under protection, diisopropylamine (0.85g, 0.0084mol) was dissolved in 10ml of tetrahydrofuran in a 50ml three-necked flask, cooled to -80°C, and butyl lithium (3.36ml, 0.0084mol) was added to keep the temperature below -60°C and stirred for 30 Minutes to prepare the LDA solution. Compound 2 (1 g, 0.0042 mol) was dissolved in 10 ml of tetrahydrofuran, and added to the prepared LDA solution, and the reaction was complete after 3 hours. Add 5ml NH 4 Quench the reaction with Cl, adjust the pH value to 3 with HCl, add 10ml of ethyl acetate to separate the liquid, wash the organic phase with water (10ml*3), dry, evaporate the solvent under reduced pressure, and crystallize the residue from methanol to obtain the product.
Embodiment 3
[0039] AlCl was placed in a 50ml three-necked bottle at room temperature 3 (1.7g, 0.013mol) was dissolved in 10ml of dichloromethane and cooled to 0°C. And diisopropylamine (2.8g, 0.028mol) was dissolved in 10ml of dichloromethane and AlCl was added 3In the dichloromethane solution, the temperature is controlled to be less than 20°C. Naturally rise to room temperature. Compound 2 (2 g, 0.0084 mol) was dissolved in 10 ml of dichloromethane and added to the reaction flask. After the reaction was complete, 50ml of water was added to quench, filtered, separated, dried over anhydrous sodium sulfate, the solvent was evaporated under reduced pressure, and the residue was crystallized by methanol to obtain the product.
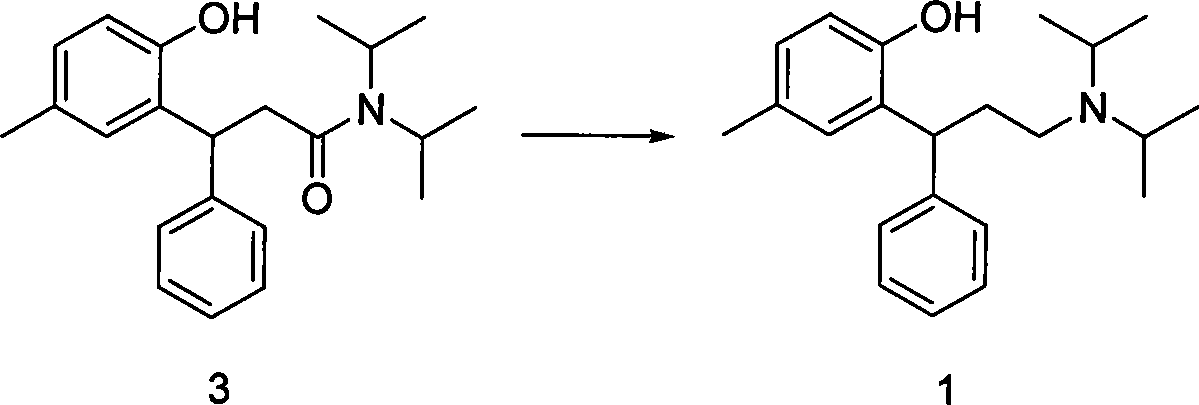
[0040] (2), the synthesis of compound 1 tolterodine free base:
PUM
| Property | Measurement | Unit |
|---|---|---|
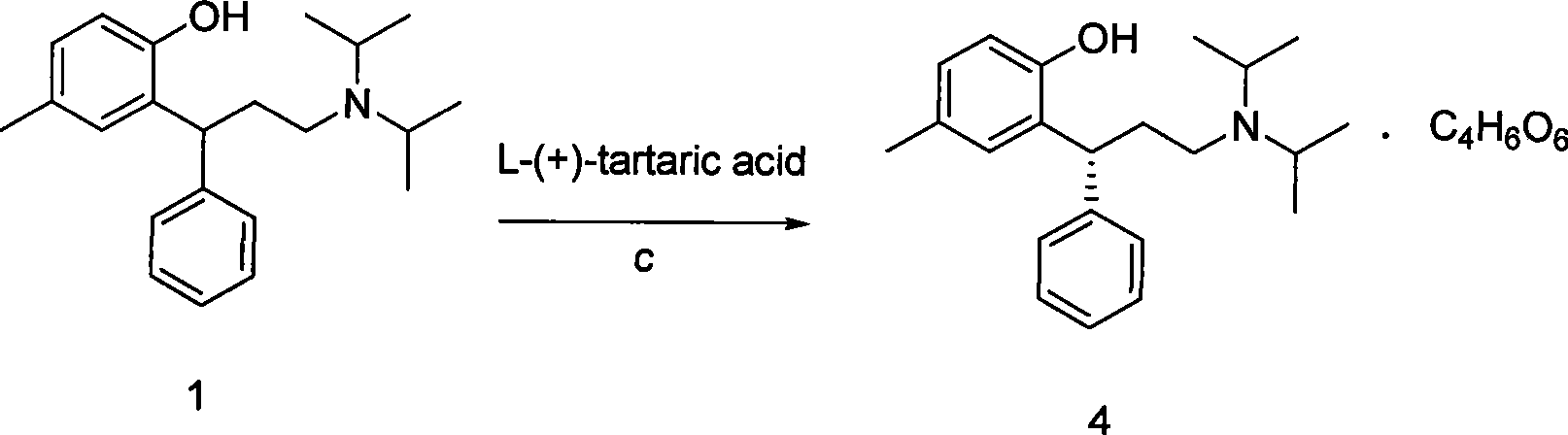
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com