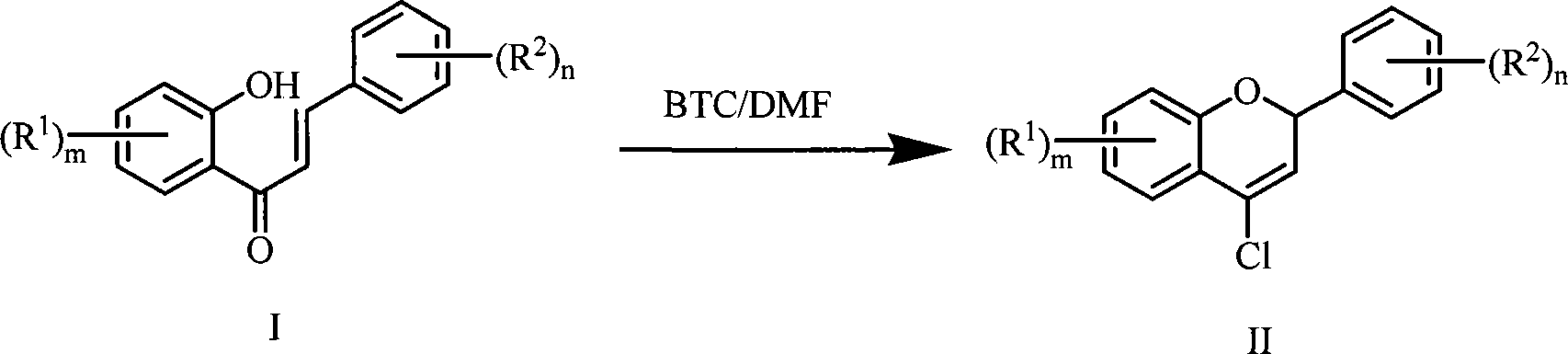
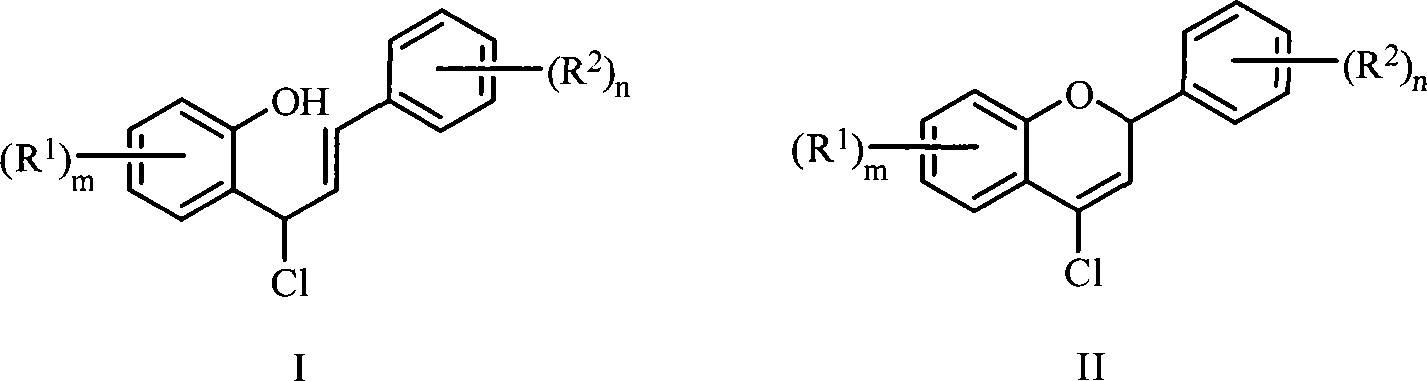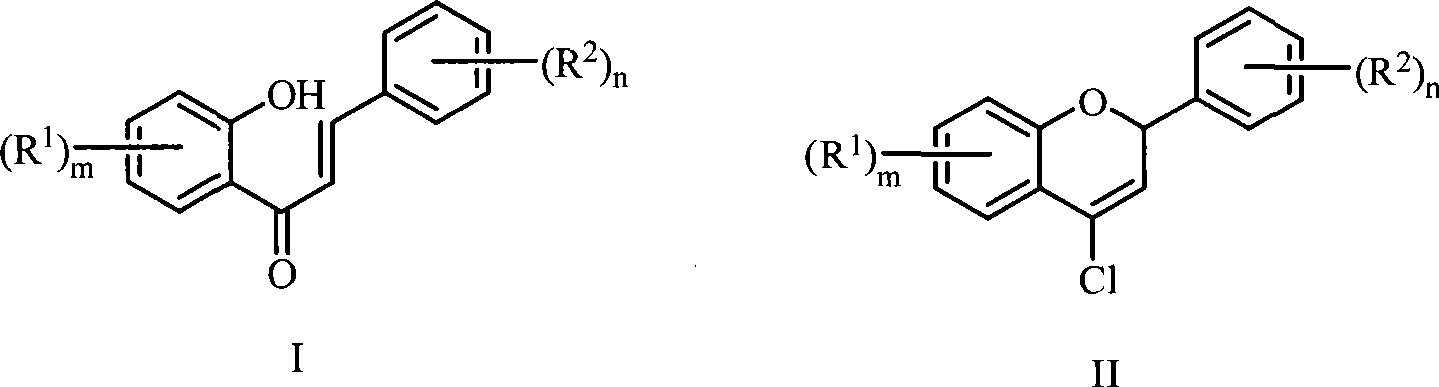Method for preparing 4-chlorine-2H-chromene derivative
A technology for derivatives and chromene, applied in the field of preparation of 4-chloro-2H-chromene derivatives, can solve the problems of reduced yield, increased side reactions, insufficient selectivity, etc., and achieves improved reaction yield and improved operation. The effect of simplicity and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0024] Example 14 Preparation of Chloro-2-phenyl-2H-chromene
[0025] Add 12mL N,N-dimethylformamide and 10mL toluene into a 150mL three-necked reaction flask equipped with magnetic stirring, drying tube, thermometer and dropping funnel, stir and cool to 0-5℃ under ice water bath, then add dropwise Bis(trichloromethyl) carbonate solution (5.94g, dissolved in 15.0mL toluene), react at 0-5℃ for 1 hour after dripping, and then add dropwise containing 1-(2-hydroxyl at 0-5℃ Benzene)-3-phenylchalcone in toluene solution (1.12g, 5mmol, dissolved in 20mL toluene), after dripping, react at 0-5°C for 1 hour, and then heat to 75-85°C for 5 hours. TLC tracks the progress of the reaction. After the reaction, the reaction product was separated and purified: the above reaction mixture was poured into 100g of crushed ice, stirred for 0.5h to complete the hydrolysis, then the organic layer was separated, the aqueous layer was extracted twice with dichloromethane 30mL×2, and the organic layers were...
Embodiment 24
[0027] Example 24-Preparation of chloro-2phenyl-2H-chromene
[0028] Add 2.31mL N,N-dimethylformamide and 5mL toluene into a 150mL three-necked reaction flask equipped with magnetic stirring, drying tube, thermometer and dropping funnel. Stir and cool down to 0-5℃ under ice-water bath, and then drip Add bis(trichloromethyl) carbonate solution (2.97g, dissolved in 5.0mL toluene), after dripping, react at 0-5℃ for 0.5 hours, then add dropwise containing 1-(2- Hydroxybenzene)-3-phenylchalcone in toluene solution (1.12g, 5mmol, dissolved in 12.4mL toluene), after dripping, react at 0-5°C for 0.5 hours, then heat to 75-85°C for reaction 1 hour. Other purification operations were the same as in Example 1, and 0.51 g of 4-chloro-2phenyl-2H-chromene was isolated and the yield was 42%.
Embodiment 34
[0029] Example 34 Preparation of Chloro-2-phenyl-2H-chromene
[0030] Add 2.31mL N,N-dimethylformamide and 8mL toluene into a 150mL three-necked reaction flask equipped with magnetic stirring, drying tube, thermometer and dropping funnel, stir and cool to 0-5℃ under ice water bath, then drop Add bis(trichloromethyl) carbonate solution (2.97g, dissolved in 10.0mL toluene), after dripping, react at 0-5℃ for 0.5 hours, then add dropwise containing 1-(2- Hydroxybenzene)-3-phenylchalcone in toluene solution (1.12g, 5mmol, dissolved in 10.0mL toluene), after dripping, react at 0-5°C for 0.5 hours, and then heat to 75-85°C for reaction 10 hour. Other purification operations were the same as in Example 1, and 0.70 g of 4-chloro-2phenyl-2H-chromene was isolated and the yield was 58%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com