Two-photon fluorescent probe for detecting Cys as well as preparation method and application thereof
A two-photon fluorescence and probe technology, used in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve problems such as lack of deep penetration, avoid phototoxicity, improve fluorescence intensity, and have wide applications. Foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of embodiment 1 Co-Cys fluorescent probe
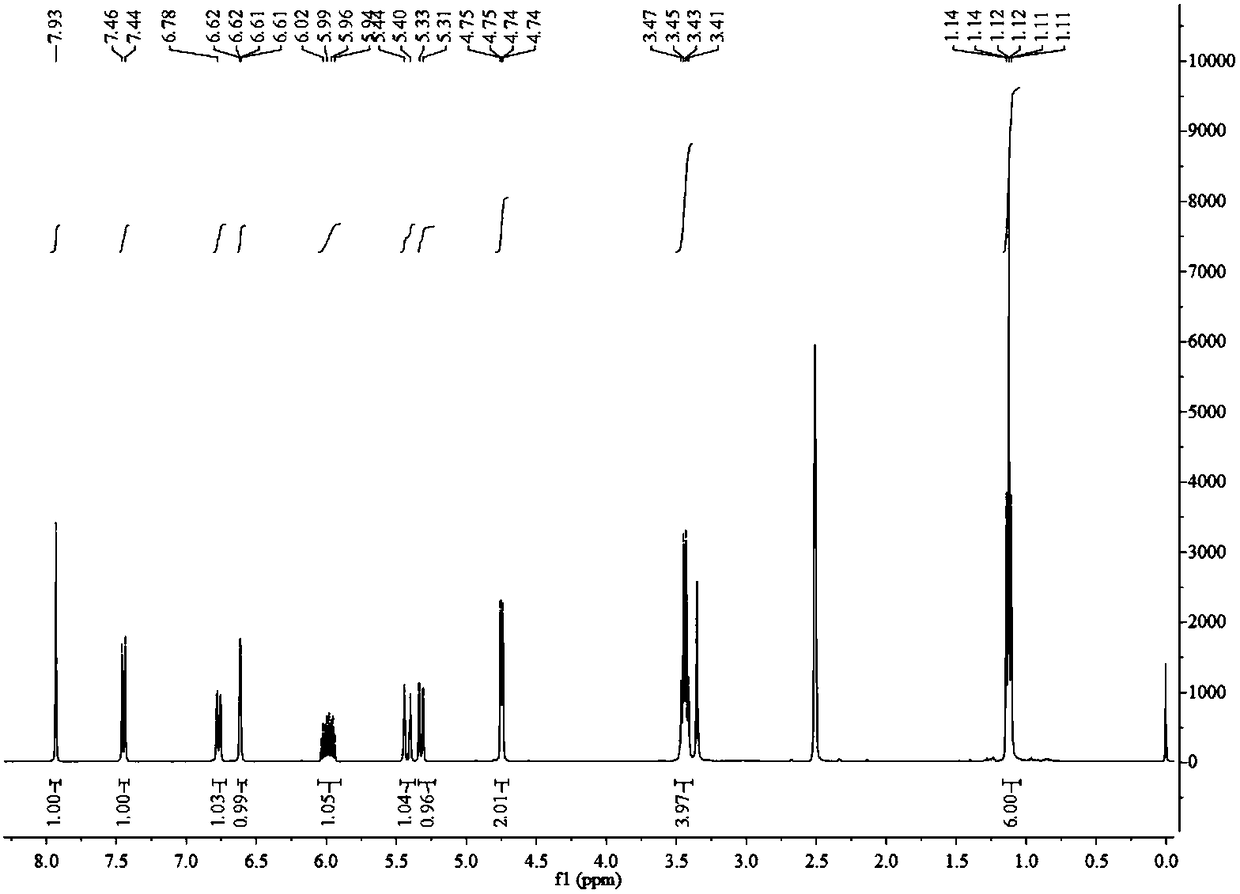
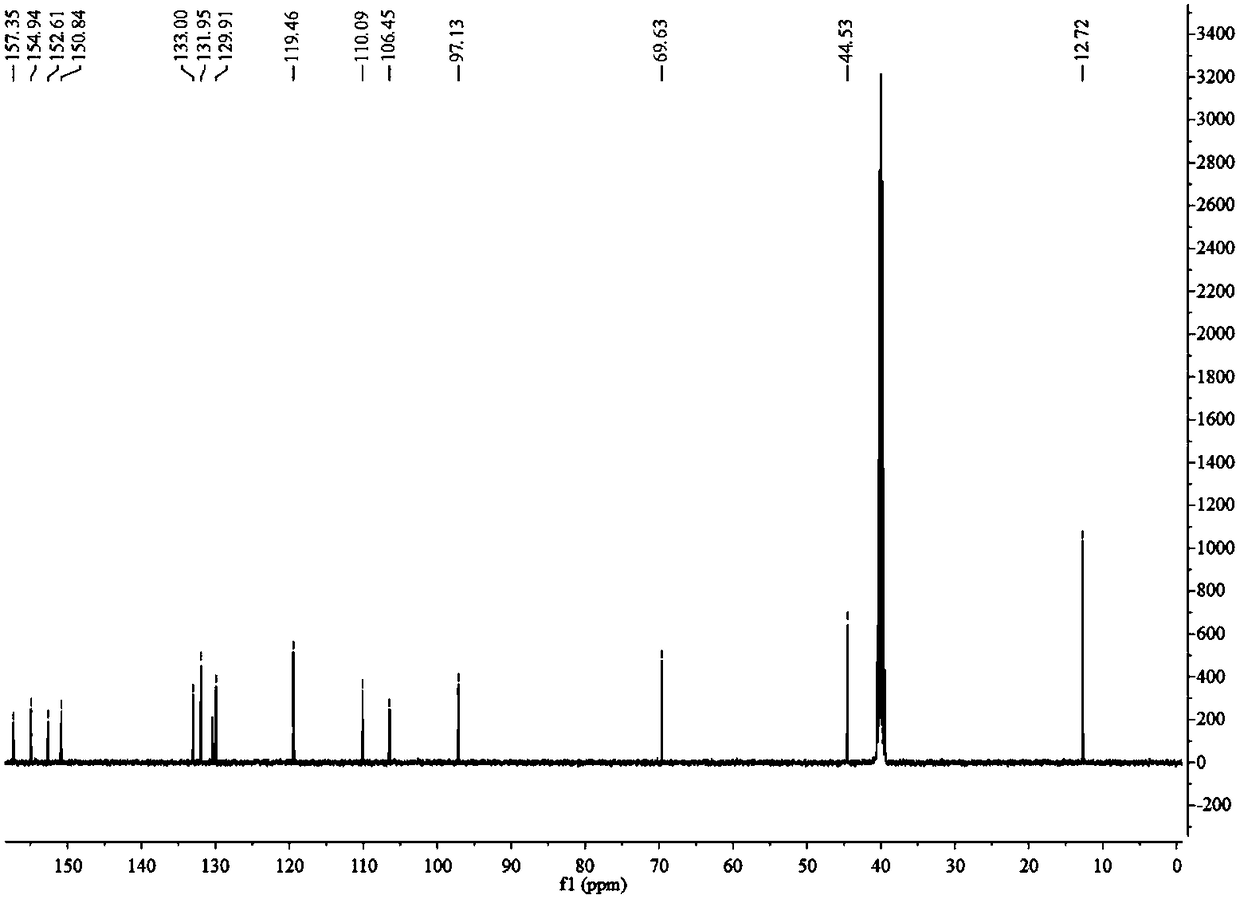
[0039] (1) In a 100 mL round bottom flask, add 1 mmol of 7-diethylamino-3-aminocoumarin (1), heat to 100°C in 20 mL of 1.5 mol / L hydrochloric acid aqueous solution, after the reaction is completed Suction filtration, the filter cake is 7-diethylamino-3-hydroxycoumarin (2). Wherein the hydrochloric acid aqueous solution is; The reaction time is 4 hours, productive rate: 91%; 1 H NMR (400 MHz, DMSO- d 6 ) δ9.51 (s, 1H), 7.29 (d, J =8,8 Hz, 1H), 7.02 (s, 1H), 6.65 (dd, J 1 =8.8Hz, J 2 =2.3 Hz, 1H), 6.51 (d, J =2.3 Hz, 1H), 3.38 (dd, J 1 =14.4Hz, J 2 =7.4 Hz, 5H),7.62 (t, J =7.0 Hz, 6H); 13 C NMR (126 MHz, DMSO) δ 159.50, 152.02, 148.10,137.47, 127.55, 117.53, 109.67, 108.86, 97.41, 44.33, 12.79; the product was directly carried on to the next step without purification:
[0040] ;
[0041] (2) Stir the compound 7-diethylamino-3-hydroxycoumarin (2) obtained in the previous step and acryloyl chloride (3) ...
Embodiment 2
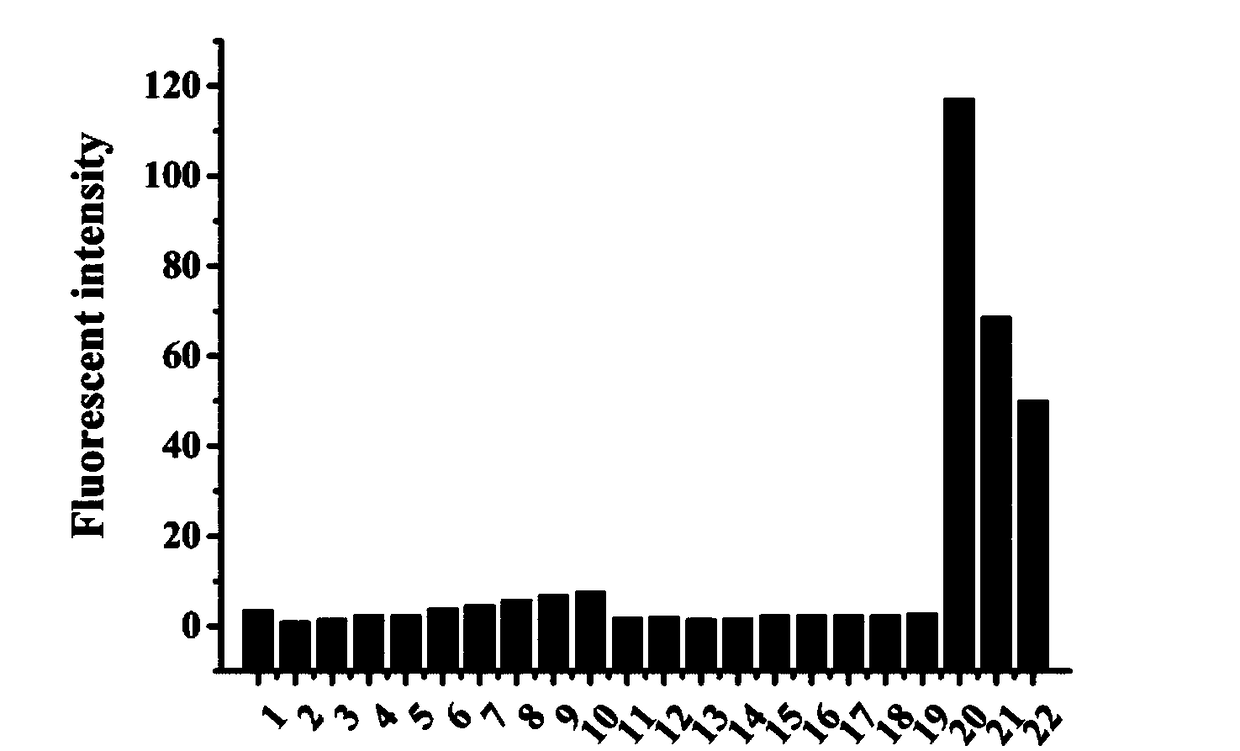
[0045] Example 2 The selectivity of Co-Cys fluorescent probes to different molecules or ions
[0046] The Co-Cys fluorescent probe in Example 1 was prepared into a mother solution with a concentration of 1 mM.
[0047] The following substances: calcium chloride, magnesium chloride, sodium nitrate, sodium nitrite, sodium bisulfite, sodium sulfide, sodium sulfate, ferrous sulfate, potassium iodide, sodium bromide, sodium hypochlorite, hydrogen peroxide, tert-butyl hydroperoxide , sodium pyruvate, formaldehyde, acetaldehyde, chloral, glyoxal, Cys, Hcy and GSH were prepared in phosphate buffer (0.01 mM, pH=7.4) to prepare 5 mL of a stock solution with a concentration of 40 mM.
[0048] Take 22 test tubes, add 25 μL probe mother solution, 1 mL DMSO and the mother solution of each ion or molecule, and control with an equal amount of water instead of interfering substances; dilute to 5 mL with phosphate buffer (0.01 mM, pH=7.4) , so that the final concentration of interfering substa...
Embodiment 3
[0049] Example 3 Fluorescence intensity of Co-Cys under different concentrations of Cys
[0050] Prepare 10 mL of stock solution with a concentration of 100 mM Cys, and dilute with water to a total of 18 concentrations of 0-55 μM, using water as a control. Dilute the Co-Cys mother solution in Example 2 to 5 μM, add different concentrations of Cys respectively, and perform fluorescence detection (λex = 400 nm, λem = 496 nm) after reacting for 50 min to detect the fluorescence intensity in each system, and use the fluorescence intensity -Cys concentration curve, such as Figure 4 shown. It can be seen from the figure that with the increase of Cys concentration, the fluorescence intensity of the reaction system increases. When the Cys concentration is 2.5-50 μM, there is a good fluorescence intensity-concentration linear relationship; when the Cys concentration reaches 55 μM, the fluorescence intensity of the reaction system reaches saturation. state.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com