Enzymatic synthesis method for fenpyroxim
A technology of enzymatic synthesis of cyhalofop-ethyl, which is applied in the field of enzyme-catalyzed synthetic herbicide cyhalofop-ethyl, herbicide enzymatic synthesis, can solve the problems of shortening the synthesis process, optical purity and total yield decline, etc. Achieve the effects of shortening the synthesis process, no by-product formation, and preventing ester hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
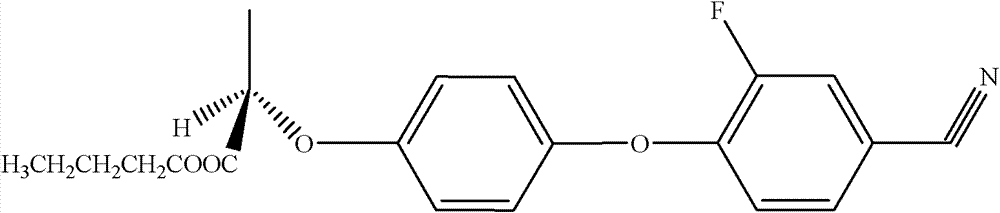
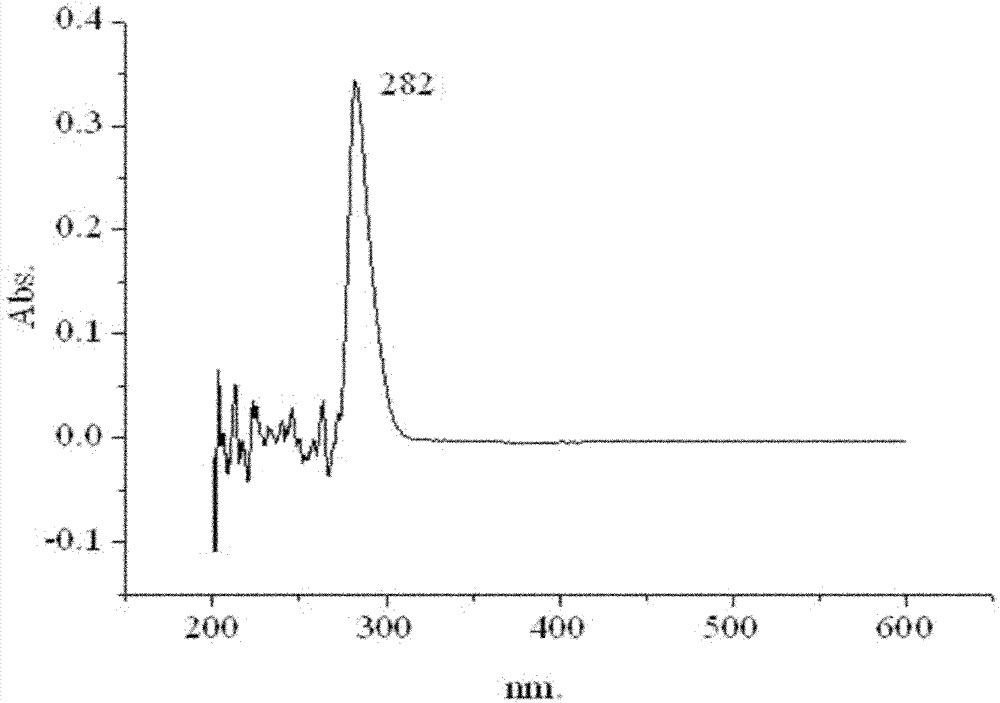
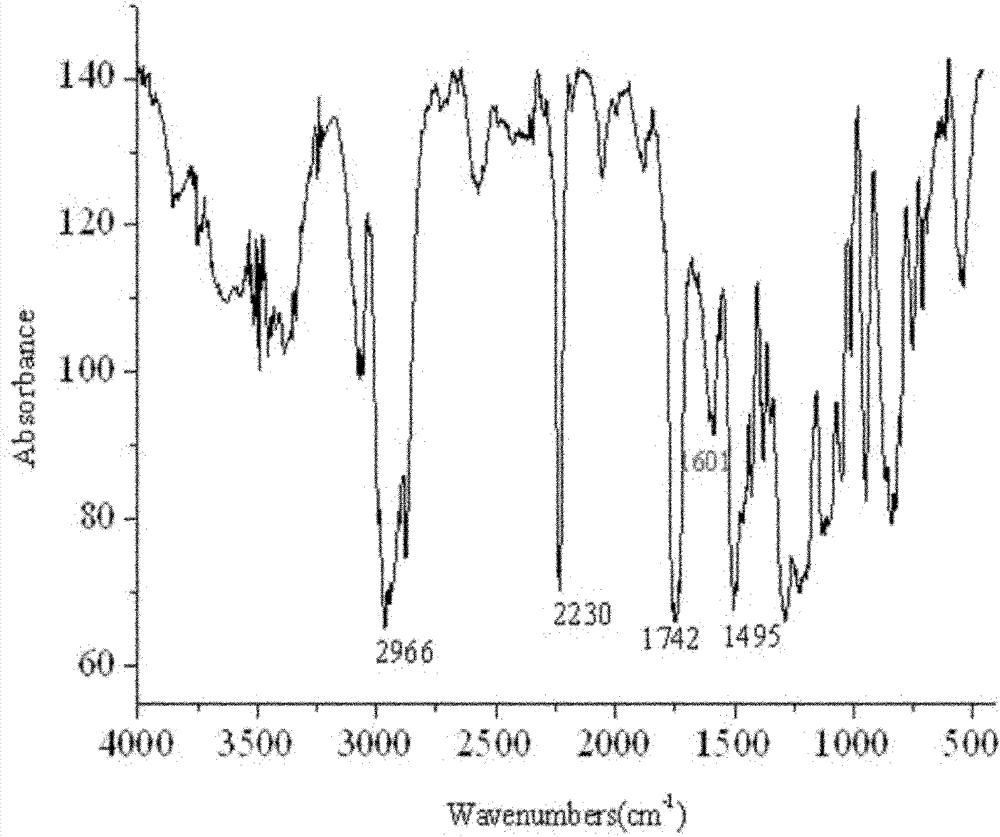
[0030] Add 0.494g (1.5mmol) ethyl (R)-2-[4-(4-cyano-2-fluorophenoxy)phenoxy]propionate, 1mL n-butanol, 9mL Acetonitrile and 500 U of Novozym 435 were reacted for 8 hours at a temperature of 60° C., a shaking speed of 200 rpm, and normal pressure, and the product was obtained by column separation. Reversed-phase high-performance liquid chromatography was adopted under the following detection conditions: a Symmetry C18 column (5 μm, 4.6 mm i.d. × 150 mm), with V (methanol): V (water) = 80: 20 as the mobile phase, and a flow rate of 1.0 mL / min, the ultraviolet detection wavelength is 282nm, the column temperature is 30°C, and the injection volume is 20μL, and the detection and analysis are carried out; the analysis shows that the product yield reaches 93.4%, and no by-products are formed. It was confirmed by infrared spectrum and nuclear magnetic resonance that the product was cyhalofop-ethyl.
Embodiment 2
[0032] Add 0.494g (1.5mmol) ethyl (R)-2-[4-(4-cyano-2-fluorophenoxy)phenoxy]propionate, 9mL n-butanol, 1mL Acetonitrile and 100 U of Novozym 435 were reacted for 8 hours at a temperature of 40°C, a shaking speed of 200 rpm, and normal pressure, and cyhalofop was obtained by column separation with a product yield of 78.1%.
Embodiment 3
[0034] Add 0.494g (1.5mmol) ethyl (R)-2-[4-(4-cyano-2-fluorophenoxy)phenoxy]propionate, 10mL n-butanol and 500U of Novozym 435 was reacted for 12 hours at a temperature of 60°C, an oscillation speed of 200 rpm, and normal pressure, and cyhalofop was obtained by column separation, and the yield of the product reached 81.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com