Dust removing device with air curtain separation and self-circulation purification
A self-circulation and air curtain technology, which is applied in the direction of transportation and packaging, packaging, bottling machines, etc., can solve problems such as interference, clean area purification air conditioning system interference, and the environmental cleanliness of the pharmaceutical processing area is difficult to meet the standard requirements, so as to avoid Effects of pollution and disturbance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
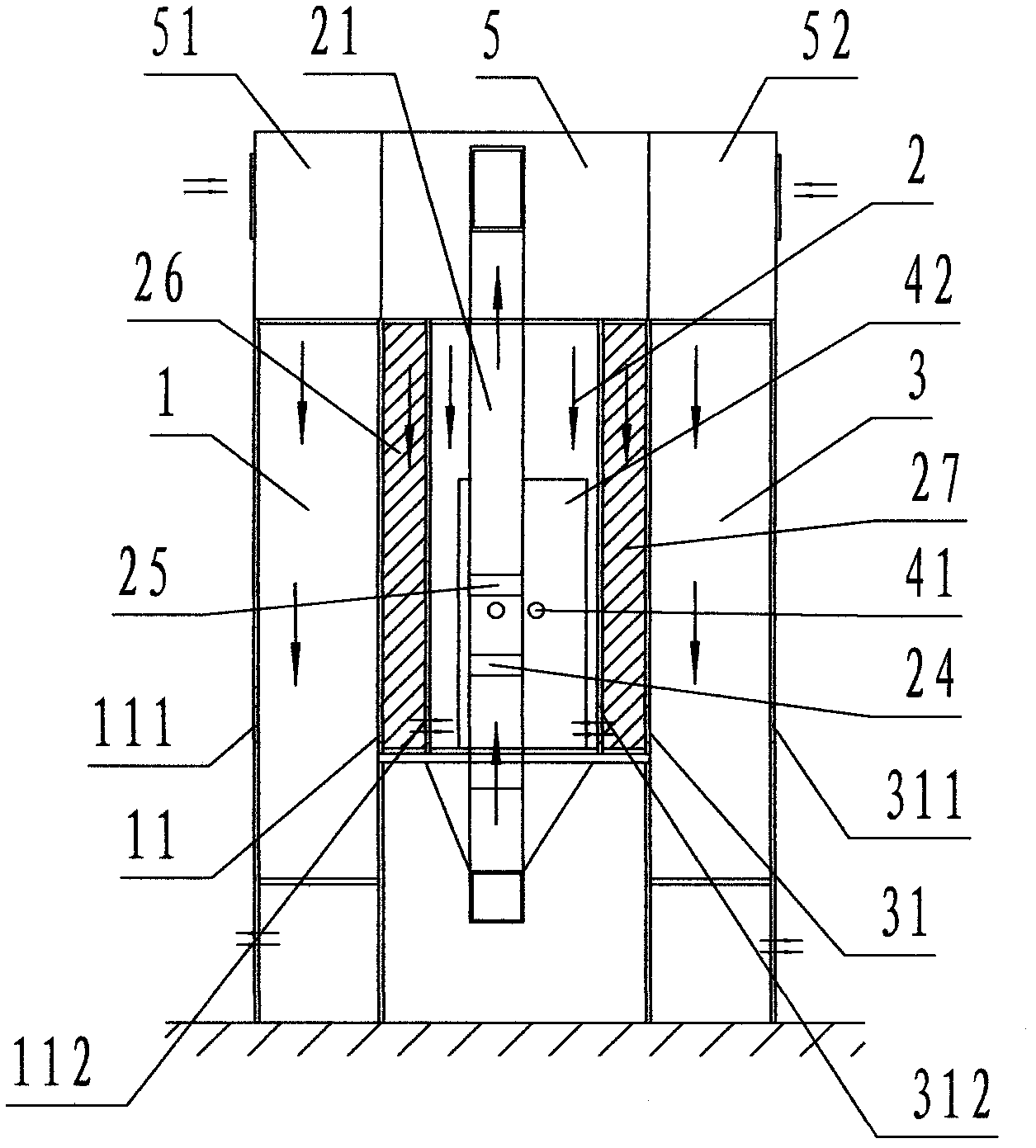
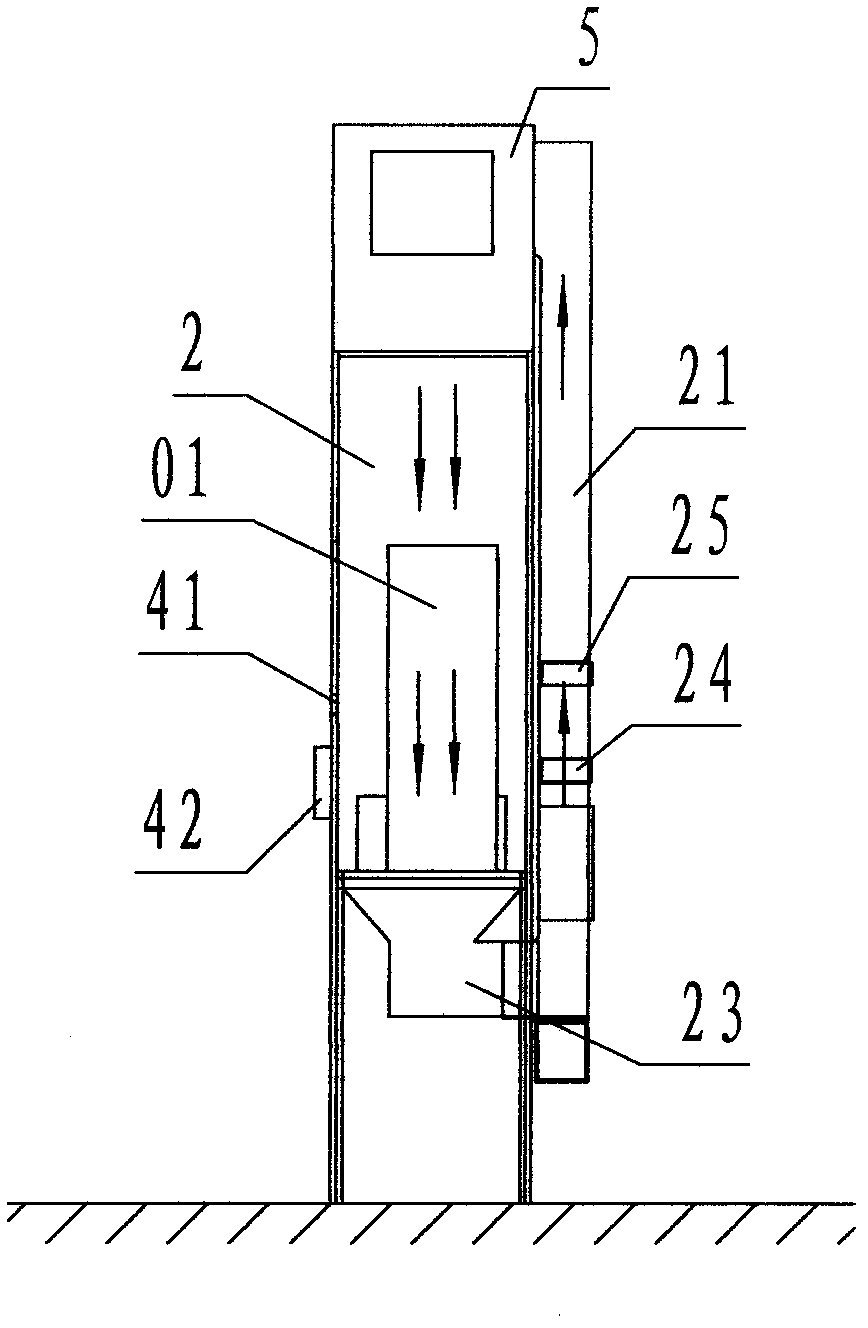
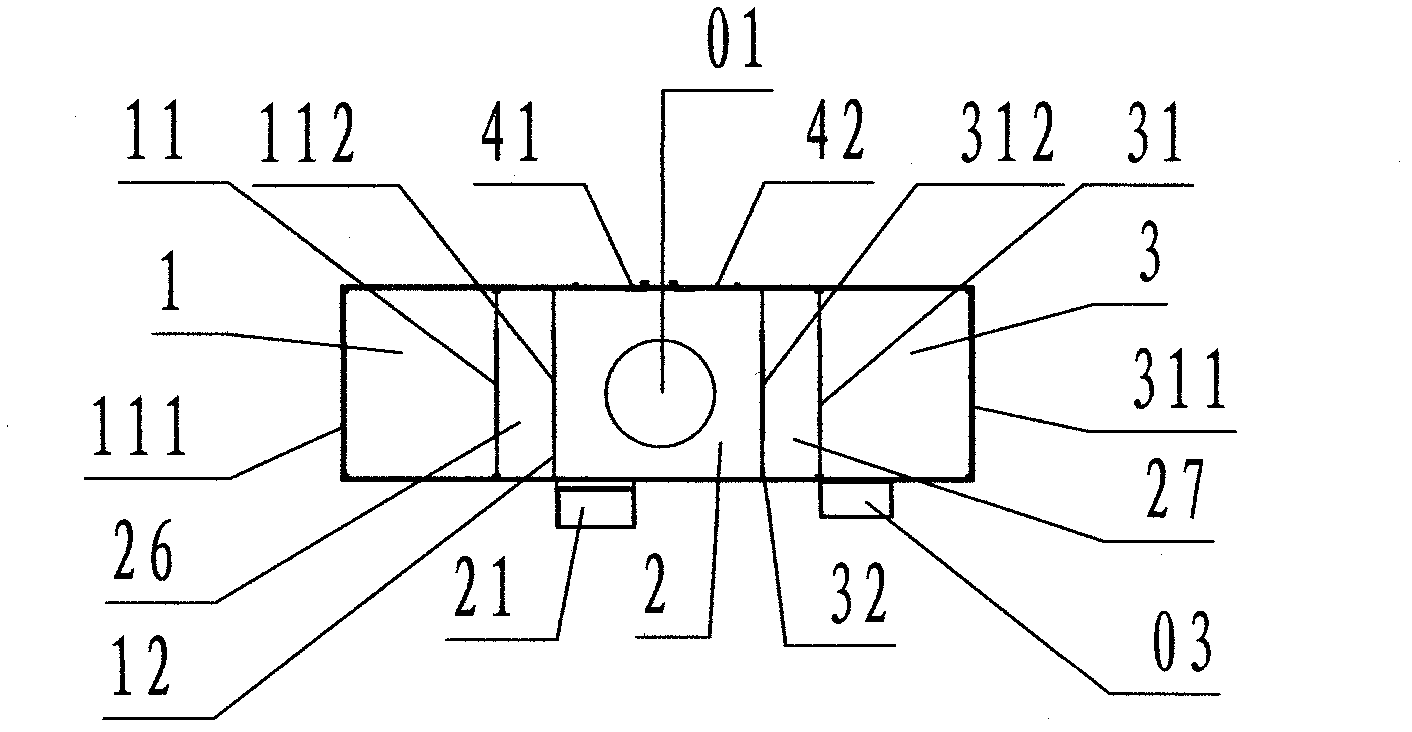
[0030] Refer to Figure 1 ~ Figure 3 , An air curtain isolation and self-circulation purification dust elimination device of the present invention includes a material input transfer cabin 1, a main isolation cabin 2, a material output transfer cabin 3, and a control cabinet 03, wherein: the main isolation cabin 2 In order to house the isolation processing equipment 01, a rectangular cabin enclosed by partition walls; the outer left side wall of the main isolation cabin 2 is provided with the material input transfer cabin 1 and the material input port 11 and the material input transfer The cabin 1 is connected; the outer right side wall of the main isolation cabin 2 is provided with the material output transfer cabin 3, and the material output port 31 is connected with the material output transfer cabin 3;
[0031] The material input transfer cabin 1 is a rectangular cabin located on the left side of the main isolation cabin 2, and the front, rear, and left sides are enclosed by p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com