Purified immunoglobulin fusion proteins and methods of their purification
A technology of immunoglobulin and fusion protein, which is applied to the preparation method of peptide, chemical equipment and method, fusion polypeptide, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
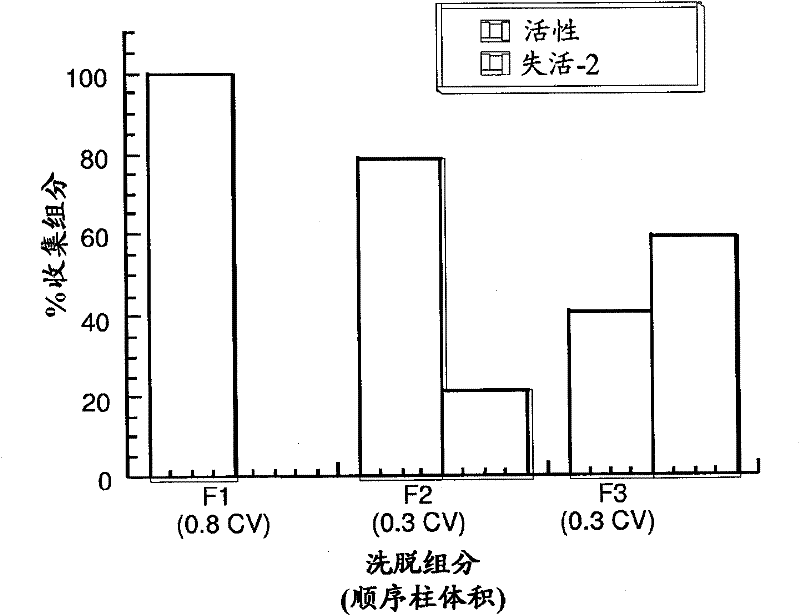
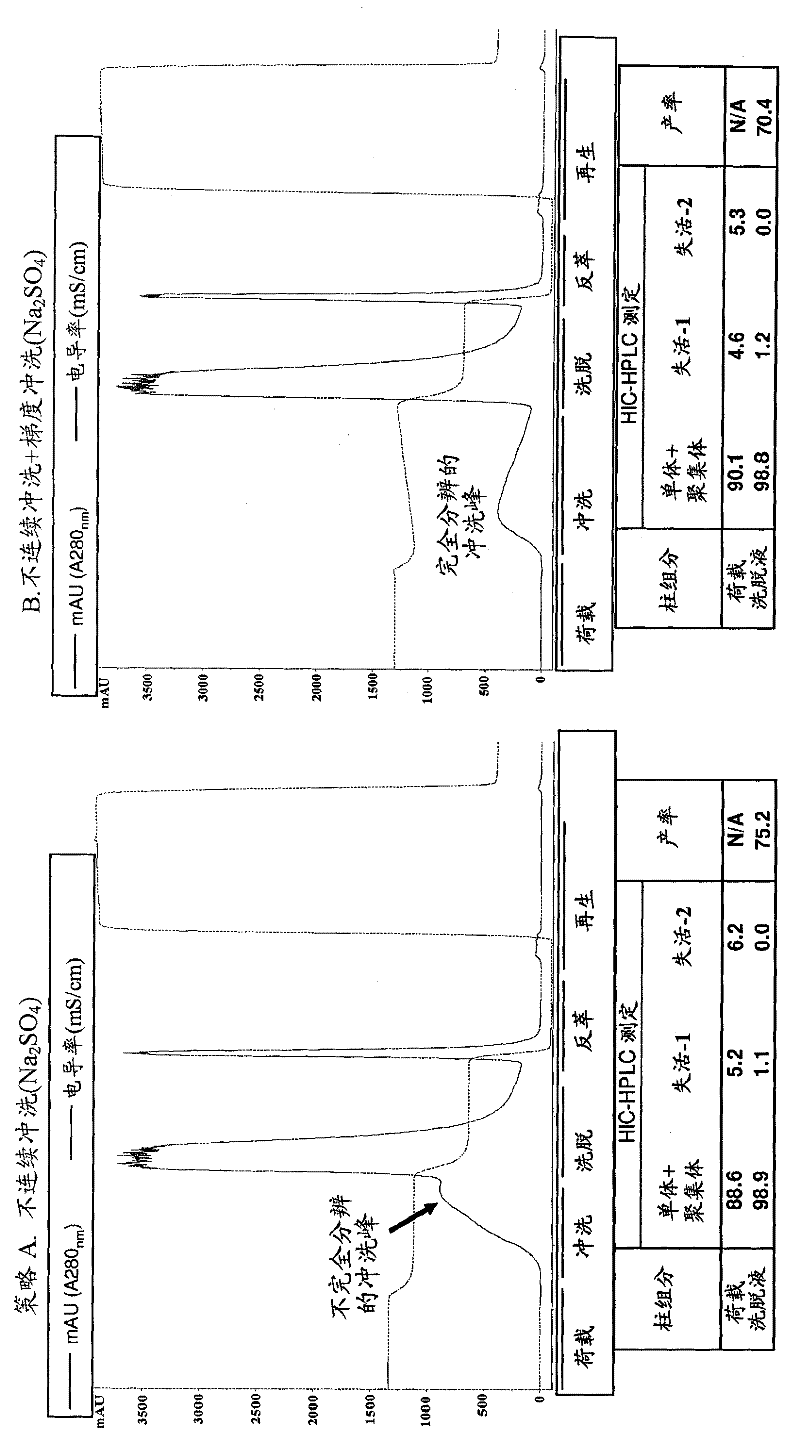
[0212] Example 1: Development of a High Resolution Hydrophobic Interaction Chromatography Method Capable of Removing Multiple Product-Related Impurities During the Purification of Antibody-Based Biologicals (Cycle 1)
[0213] For Phase 1 clinical production, a HIC process step capable of removing two monomeric but inactive product-associated impurities from the antibody-based product was developed. During subsequent development, efforts were made to improve capacity and robustness while maintaining chromatographic resolution. A development study will be described in which alternative HIC resins and binding salts are explored, and parameters critical to separation efficiency are identified through a design of experiments approach. A second HIC step with a different resolution was used downstream of the same process to remove a third product-related impurity. Issues and challenges faced in establishing robust methods suitable for production-scale clinical preparation will be di...
Embodiment 2
[0253] Example 2: Purification of Bioactive LT-β-R-IG Fusion Protein: Cycle 2
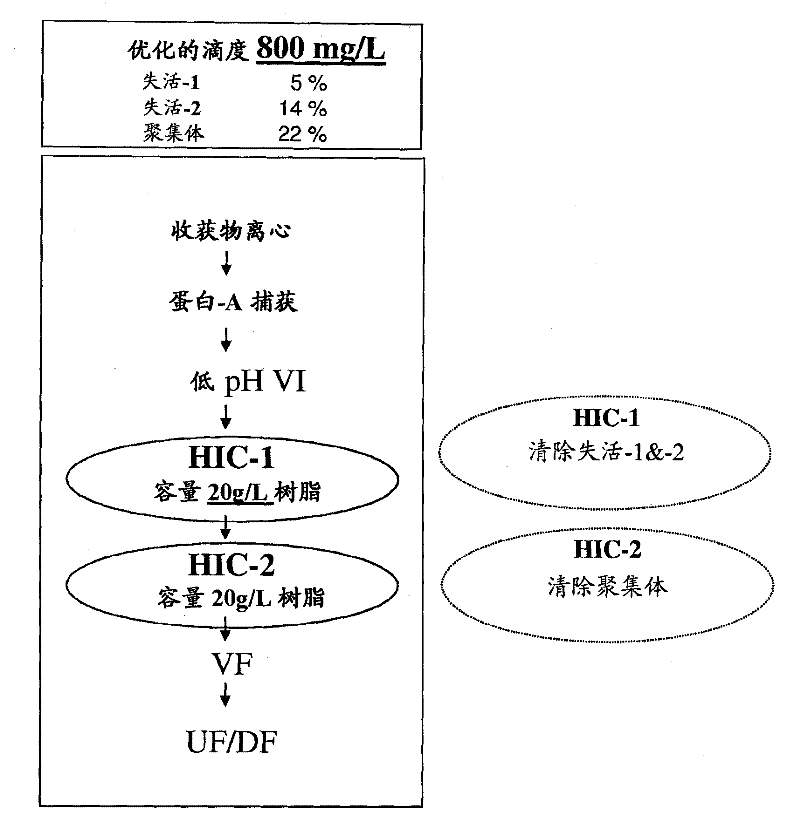
[0254] The following example describes a method of purifying an Ig-fusion biologic, the LT-β-R-Ig fusion protein, from a feedstock containing a high percentage of product-related impurities, ie about 50% product-related impurities. As noted above, the starting material for LT-β-R-Ig contains different types of impurities, namely aggregated species and two distinct, disulfide-disordered forms of the product—termed inactivation of LT-β-R— 1 and inactivated-2 forms. Because these impurities are closely associated with LT-β-R-Ig (the product), purification of the desired product poses challenges.
[0255]The production method of LT-β-R-Ig was originally developed for early clinical research and was modified to increase the production rate to a level sufficient for commercialization. To achieve this, cell culture productivity was increased by 4 times. However, this change was accompanied by an increa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com