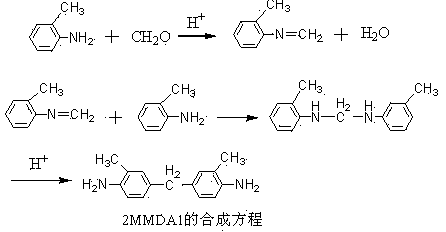
Method for synthesizing 3,3'-dimethyl-4,4'-diamidodiphenylmethane
A technology of diaminodiphenylmethane and dimethyl, which is applied in the field of synthesis of organic monomers, can solve problems such as unsatisfactory product purity, multiple recrystallizations, and reduced yields, and achieves suppression of side reactions, mild process conditions, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A method for synthesizing 3,3'-dimethyl-4,4'-diaminodiphenylmethane, comprising the following process steps:
[0034] a. In a four-necked flask equipped with a stirrer and a thermometer, add 10.7g o-toluidine, and protect it with nitrogen;
[0035] b. 36.5% of 12 g hydrochloric acid was diluted to 50ml with distilled water, the diluted hydrochloric acid was 7.3%, poured into the dropping funnel, added dropwise at a temperature of 15°C, and the dropping time was 0.5 h;
[0036] c. Add 4.06g of formaldehyde with a concentration of 37% dropwise within 0.5h, and react at the same temperature for 1h; then gradually raise the temperature to 55°C within 1.5h, and react for 1h; gradually raise the temperature to 72°C within 0.5h, and react for 2h ;
[0037] d. After the reaction is over, when the temperature is naturally lowered to 35°C, add excess ammonia water dropwise until the pH of the solution is 7, then add potassium hydroxide to adjust the pH of the solution to 9, filt...
Embodiment 2
[0040] A method for synthesizing 3,3'-dimethyl-4,4'-diaminodiphenylmethane, comprising the following process steps:
[0041] a. In a four-necked flask equipped with a stirrer and a thermometer, add 10.7g o-toluidine, and protect with nitrogen;
[0042] b. Dilute 15 g hydrochloric acid of 36.5% to 70ml with distilled water, after dilution, the hydrochloric acid is 6.5%, pour it into the dropping funnel, add dropwise at a temperature of 30°C, and the adding time is 1.5 h;
[0043] c. Add 5.27g of formaldehyde with a concentration of 37% dropwise within 1.5h, and react at the same temperature for 1.5h; then gradually raise the temperature to 60°C within 1h, and react for 1.5h; gradually raise the temperature to 74°C within 0.5h, and react for 3h ;
[0044] d. After the reaction is completed, when the temperature is naturally lowered to 35°C, add excess ammonia water dropwise until the pH of the solution is 8, then add potassium hydroxide to adjust the pH of the solution to 9.5, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com